Compositions containing (s)-bethanechol and their use in the treatment of insulin resistance, type 2 diabetes, glucose intolerance and related disorders
a technology of bethanechol and enantiomer, which is applied in the field of s-bethanechol, can solve the problems of postprandial hyperglycemia, impaired glucose disposal effect, and large shifting of glucose from blood to skeletal muscle glycogen stores, and achieves greater insulin responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
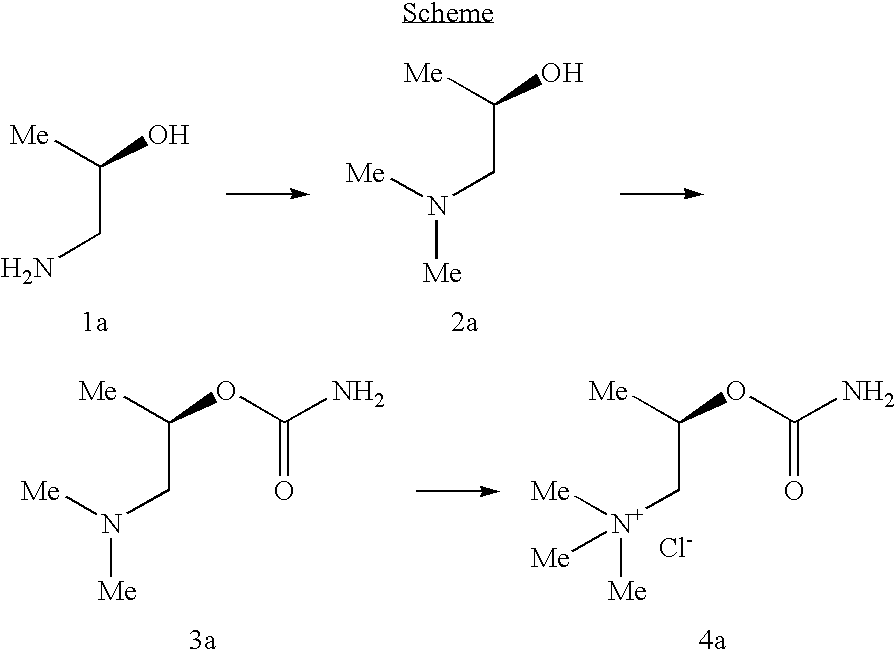
Synthesis of (R)-Bethanechol Chloride
[0114]All the preparations were carried out according to the procedures described in Micheli, C. et al, Il Farmaco—Edizione Scientifica 1983, 38(7), 514-20, plus some modifications.
[0115]The reactants (R)-(−)-1-Amino-2-propanol (1a) and (S)-(+)-1-Dimethylamino-2-propanol (2b) were commercially available from Aldrich company.
Procedures
Preparation of (R)-(−)-1-Dimethylamino-2-propanol (2a)
[0116]In a 100 mL round-bottom flask equipped with a magnetic stirring bar, and a refrigerant, (R)-(−)-1-Amino-2-propanol (3 mL, 37 mmol) was introduced under N2 atmosphere, and cooled to 0° C. in ice bath. Formic acid (7 mL, 175 mmol) was added slowly and dropwise, followed with formaldehyde (5 mL, 67 mmol). The reaction mixture was heated at reflux for overnight, allowed to cool down at room temperature, and 6N HCl(aq) (25 mL) was added. The acidic mixture was washed with CH2Cl2 (3×20 mL), basified to pH 13 with a slow adjunction of 50% NaOH(aq) (40 mL), and ext...
example 2
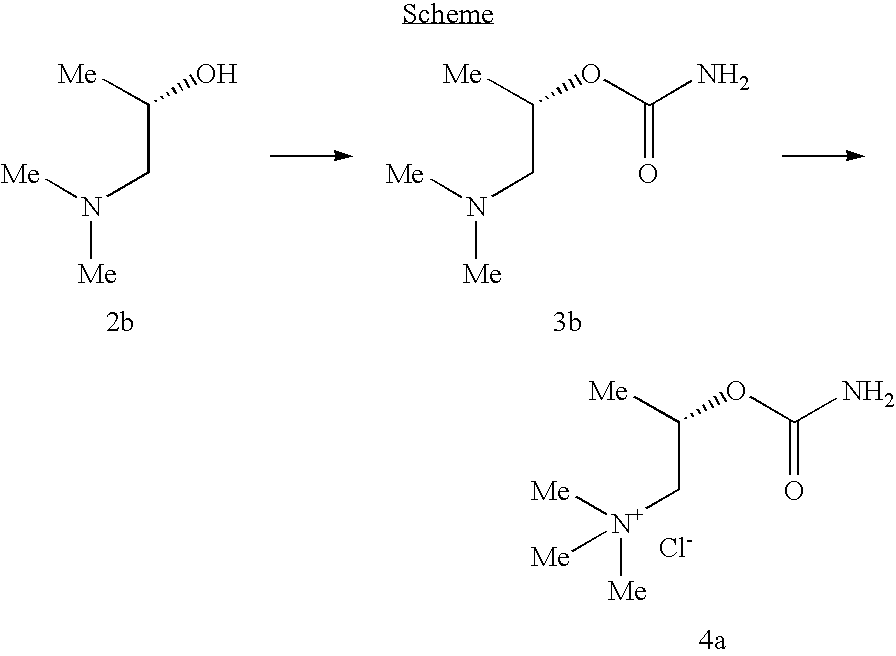
Synthesis of (S)-Bethanechol Chloride
[0119]
Procedures
Preparation of (S)-(+)-2-Carbamyloxy-1-(N,N-dimethyl)-propylamine (3b)
[0120]Compound 3b was prepared following the procedure described for the synthesis of 3a, but using commercially available (S)-(+)-1-Dimethylamino-2-propanol (Aldrich source).
Preparation of (S)-(+)-2-Carbamyloxy-1-(N,N,N-trimethyl)-propylammonium chloride (4b) ((S)-Bethanechol chloride)
[0121]Compound 3b was reacted with methyl iodide to afford product 4b, following the procedure similar for the synthesis of 4a.
example 3
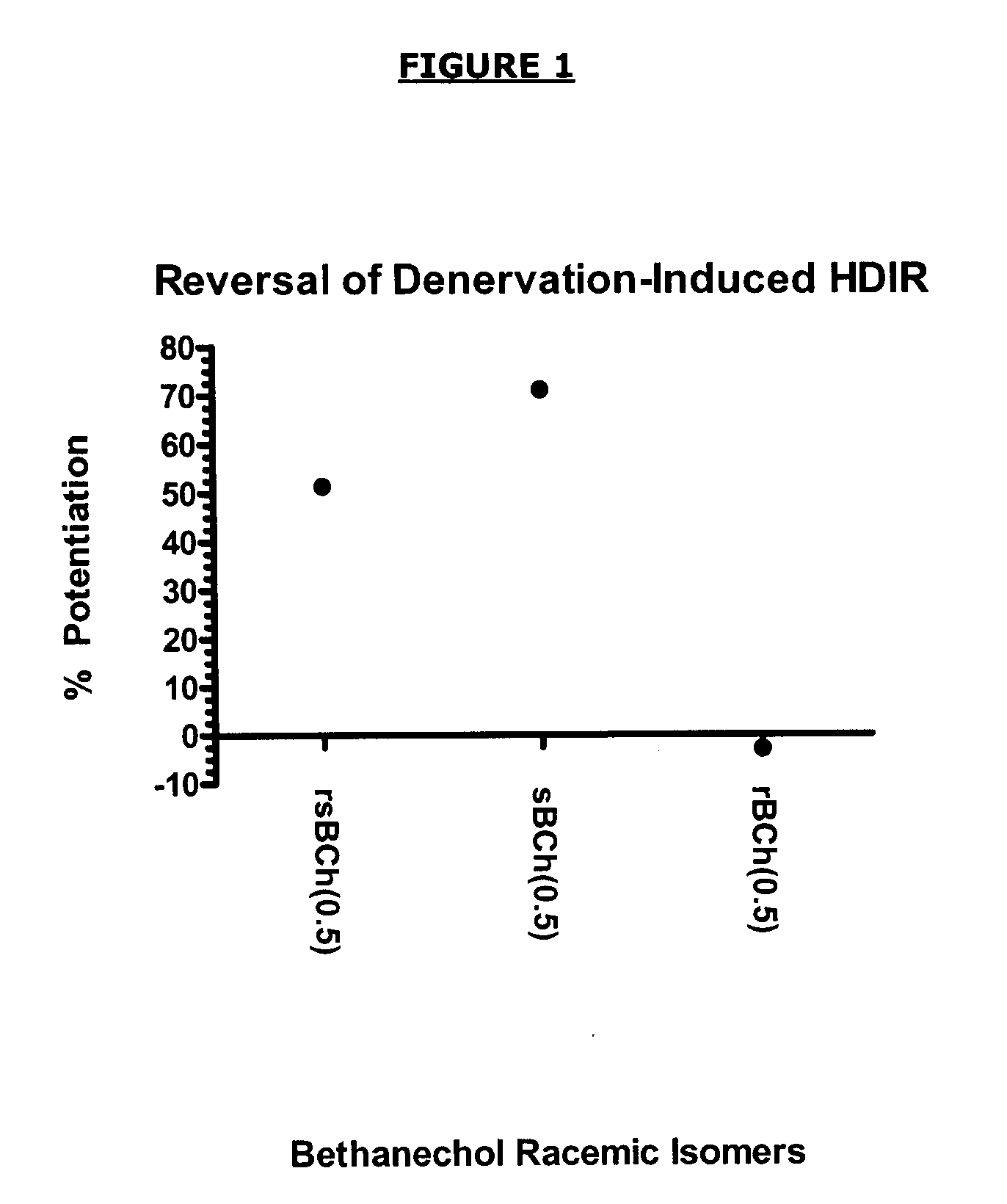
Comparison of racemic bethanechol, R-bethanechol and (S)-bethanechol Binding to Muscarinic M1 Receptors
Radioligand Binding Muscarinic M1 Binding Assay
[0122]The binding assay methodology was an adaptation of the methodology set out in Buckley N J, Bonner T I, Buckley C M and Brann M R (1989), Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 35(4): 469-476 and Luthin G R and Wolfe B B (1984), Comparison of [3H]pirenzepine and [3H]quinuclidinylbenzilate binding to muscarine cholinergic receptors in rat brain. J Pharmacol Exp Ther. 228(3):648-655.
[0123]The binding assay was performed under the following conditions:
Source: human recombinant CHO cells
Ligand: 0.8 nM [3H] N-Methylscopolamine
Vehicle: 1% DMSO
[0124]Incubation Time / Temp: 2 hours at 25° C.
Incubation Buffer: 50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 1 mM EDTA
Non-specific Ligand: 1 μM Atropine
KD: 0.26 nM
[0125]Bmax: 2 μmol / mg protein
Specific Binding: 95%
[0126]Quantification Meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com