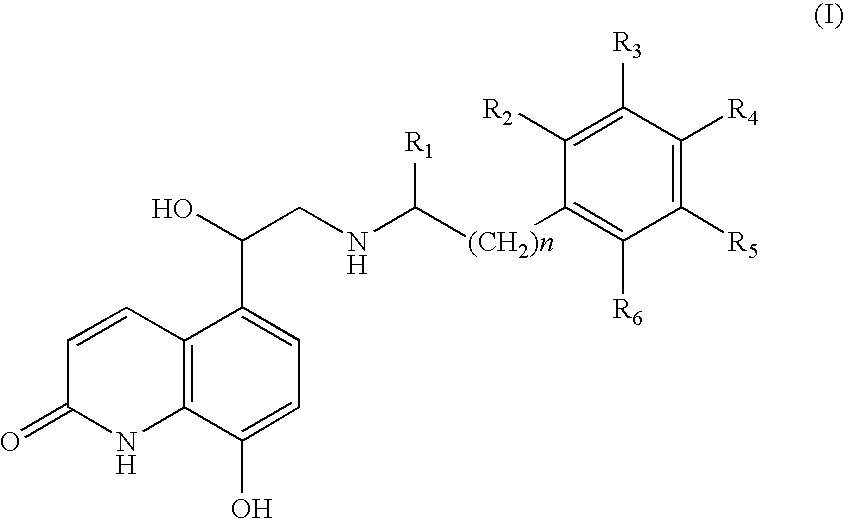
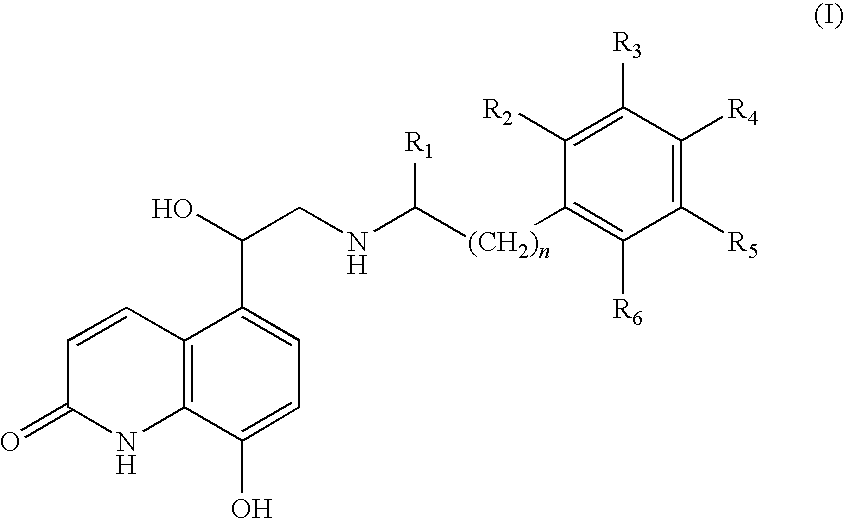
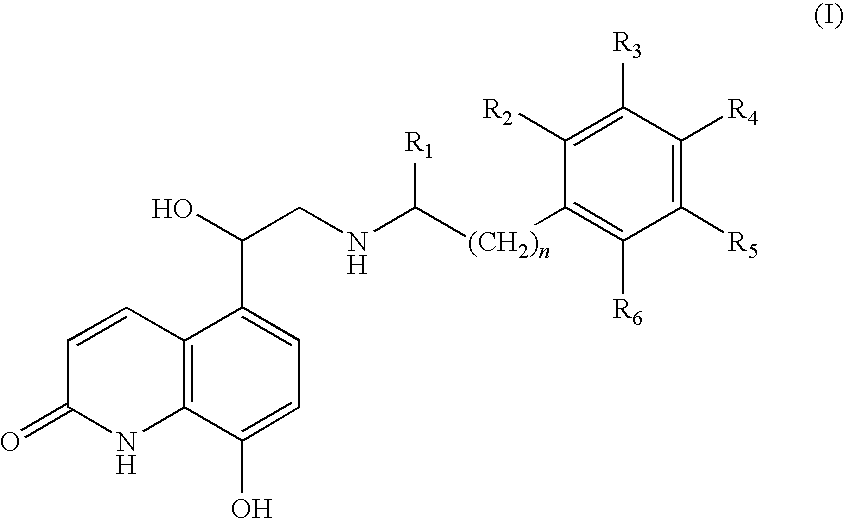
Pressurised metered dose inhalers containing solutions of beta-2 agonists
a technology of beta-2 agonists and inhalers, which is applied in the direction of drug compositions, dispersed delivery, aerosol delivery, etc., can solve the problems of mucosal damage, irreversible narrowing of airways and fibrosis of lung tissue, and poor understanding of pathology, so as to improve the effect of asthma control, enhance the beneficial effects of the other, and increase the expression of 2-receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Superfine TA 2005 HFA Formulations
[0094]A formulation for delivering a nominal dose of 1 μg per actuation of active ingredient was prepared with the composition as follows:
AmountsPer unitNominalComponentsmg%dose μgTA 20050.150.0016w / v1Ethanol165015w / w—HCl 0.1 M2.0*0.018w / w—HFA 134a q.s. to 9.45 ml9347.85——*equivalent to 2.0 μl
[0095]The formulation (120 actuations / canister, overage of 30 actuations) was filled in aluminum canisters having the internal surface coated with TEFLON® (two stage pressure filling) and fitted with a metering valve having a 63 μl metering chamber. An actuator with an orifice diameter of 0.22 mm was used. Results were obtained as a mean of 2 cans.
[0096]Analogously, formulations able of delivering a nominal dose of 2, 3 or 4 μg per actuation of active ingredient can be prepared. The aerodynamic particle size distribution was measured by ACI, according to page 16 lines 10 to 18 and the delivery characteristics of each formulation were determined in terms of the ...
example 2
Superfine HFA Formulation Comprising TA 2005 and 22R-Budesonide
[0101]A formulation for delivering, respectively, a nominal dose of 1 μg of TA 2005 and 80 μg of 22R-budesonide per actuation was prepared with the composition as follows:
AmountsPer unitNominalComponentsmg%dose μgTA 20050.150.0016w / v122R-budesonide12.000.127w / v80Ethanol165015w / w—HCl 0.1 M3.3*0.03w / w—Water220.052.0w / wHFA 134a q.s. to 9.45 ml9114.5——*equivalent to 3.3 μl
The formulation (120 actuations / canister, overage of 30 actuations) was filled in aluminum canisters having the internal surface coated with Teflon (two stage pressure filling) and fitted with a metering valve having a 63 μl metering chamber.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| aerodynamic diameters | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com