Methods and compositions for treating diabetes, metabolic syndrome and other conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
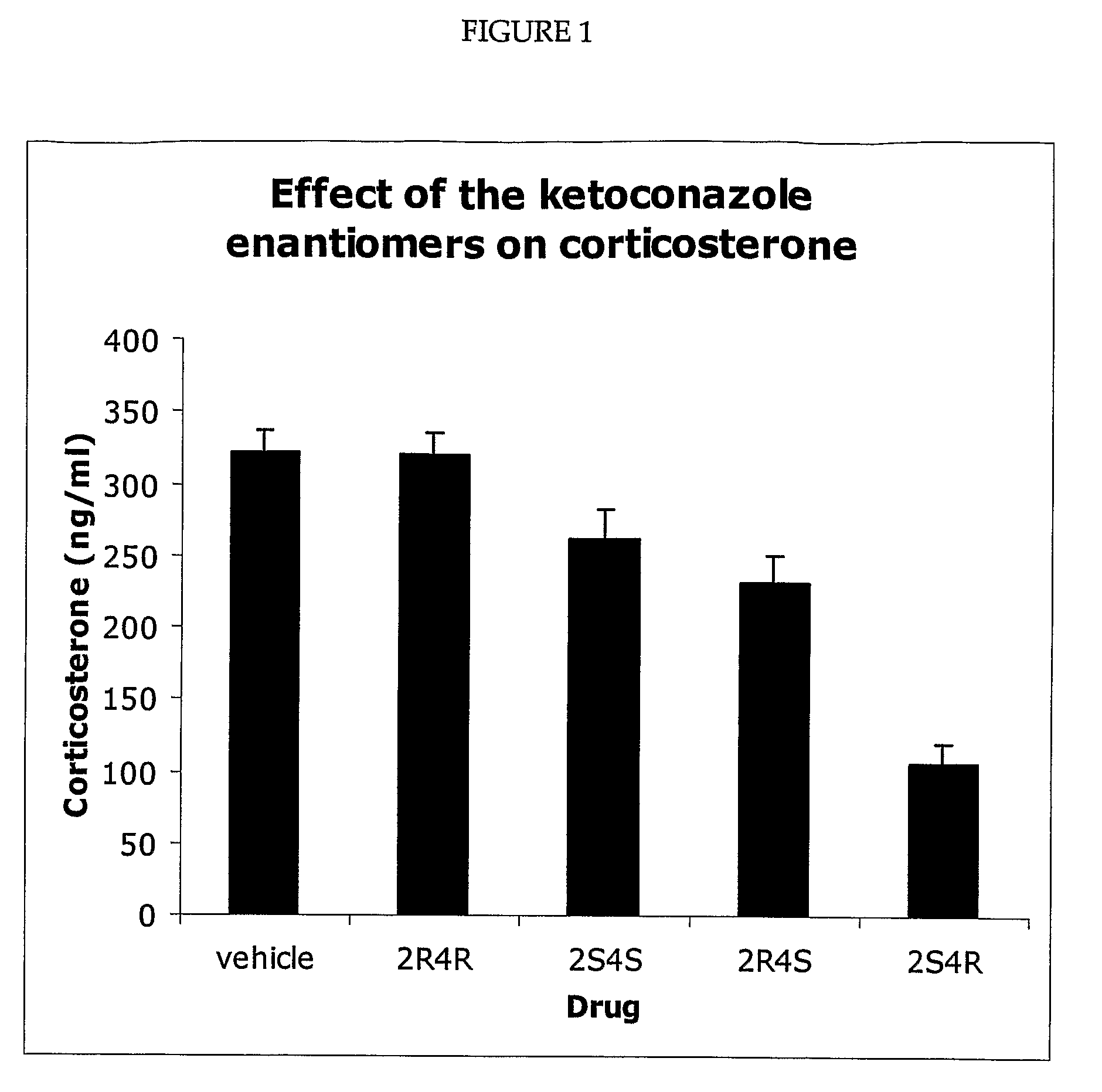
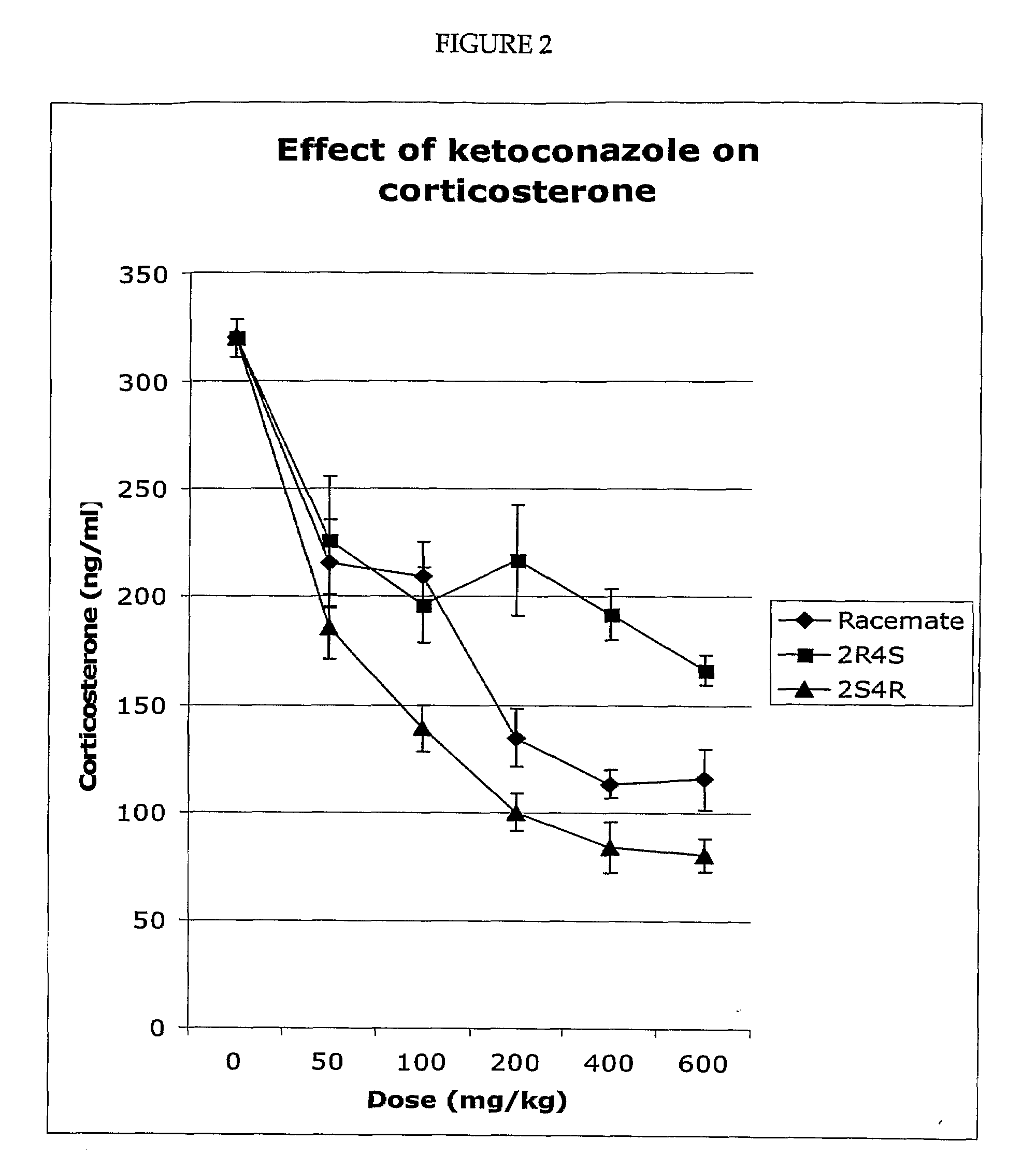
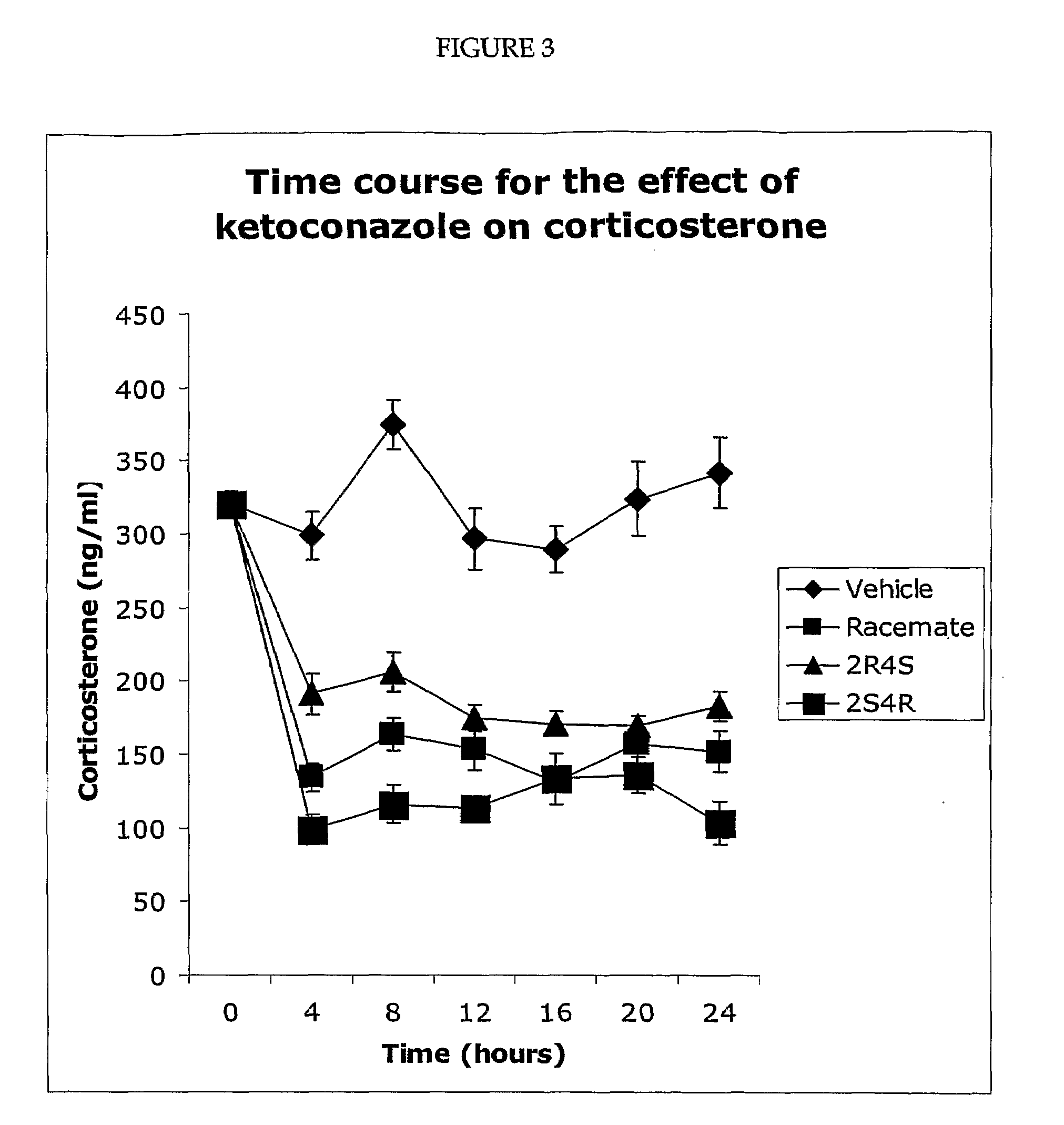
Measurement of Corticosterone and Cholesterol Following Dosing with Racemic Ketoconazole and the Enantiomers of Ketoconazole
[0098]The effect of ketoconazole and the ketoconazole enantiomers on corticosterone levels in the plasma of Sprague Dawley rats was determined. For the experiment described in FIG. 1, the four enantiomers and the racemic ketoconazole were suspended in olive oil. To generate the results shown in FIG. 1, five groups (eight per group) of rats were used. The rats were maintained on a 14 / 10 hour light / dark cycle and allowed free access to food and water. Each rat was dosed (200 mg drag / kg body weight) via a gastric tube. The rats in group 1 were dosed with the vehicle (olive oil), while the rats in the other four groups were dosed with one of the four ketoconazole enantiomers as indicated. All of the rats were dosed between 2.00 and 3.00 pm and were sacrificed four hours later (between 6.00 and 7.00 pm). Plasma was prepared and the concentration of corticosterone de...
example 2
Measurement of Drug Exposure Following Dosing with Racemic Ketoconazole and the Cis Enantiomers of Ketoconazole
[0101]In this example, dogs were treated with ketoconazole or with the 2S,4R enantiomer only, and the plasma levels of the corresponding drug were determined.
Pharmacokinetics of Racemic Ketoconazole
[0102]Two groups of three male and three female dogs per group were studied. Each dog was dosed with racemic ketoconazole, and the concentration of racemic ketoconazole in the plasma of the dogs was determined on the first day of dosing and again after four weeks of daily dosing. The two groups differed in that, in one group, the racemic ketoconazole was provided as a dry white powder in a gelatin capsule, and in the other, the racemic ketoconazole was provided as a suspension in olive oil.
[0103]The dogs were purpose bred beagle dogs obtained from Covance Research Products, Inc., Cumberland, Va. USA. The dogs were 4.5 to 5 months old at the start of dosing. The dogs were housed i...
example 3
Formulation and Clinical Trial of the 2S,4R Enantiomer Substantially Free of the 2R,4S Enantiomer of Ketoconazole in Type 2 Diabetes
A. Abbreviations
[0113]The following abbreviations are used in this Example.
Term / AbbreviationExplanationALTalanine transaminaseASTaspartate transaminaseAUCarea under the curveBidtwice dailyBiwtwice weeklyBUNblood urea nitrogenCVcoefficient of variationELISAenzyme-linked immunosorbent assayFDAFood and Drug AdministrationGIGastrointestinalGLPGood Laboratory PracticeINDInvestigational New Drug (application)IVIntravenousMedDRAMedical Dictionary for Regulatory ActivitiesNDANew Drug ApplicationNOAELno-observed-adverse-effect levelPBSphosphate-buffered salineQdDailyQwWeeklyRP-HPLCreverse-phase high-performance liquid chromatographySBASummary Basis of ApprovalSCsubcutaneous, subcutaneouslySDstandard deviationSDS-PAGEsodium dodecyl sulfate-polyacrylamide gel electrophoresisSE-HPLCsize-exclusion high-performance liquid chromatographyUSPUnited States PharmacopoeiaW...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com