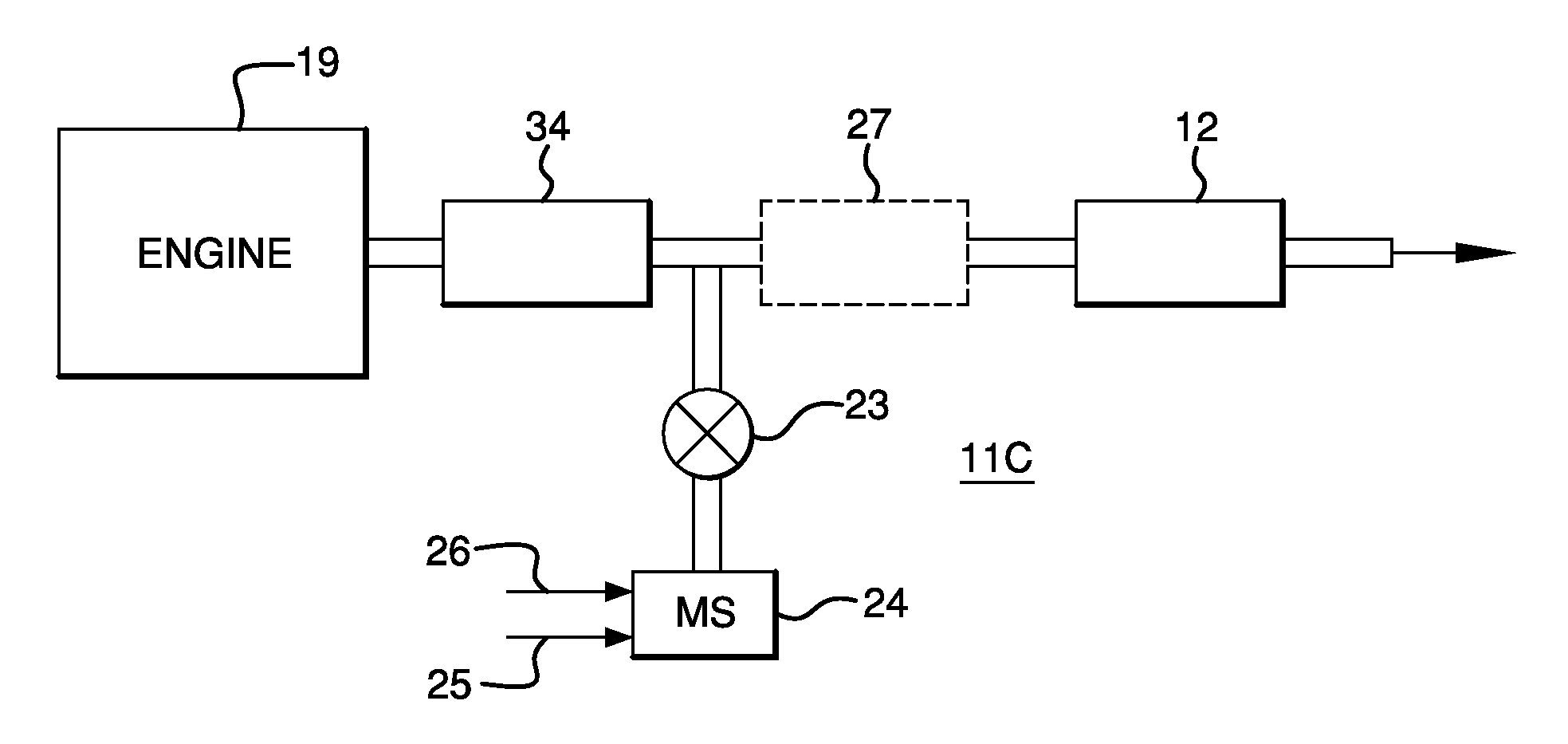
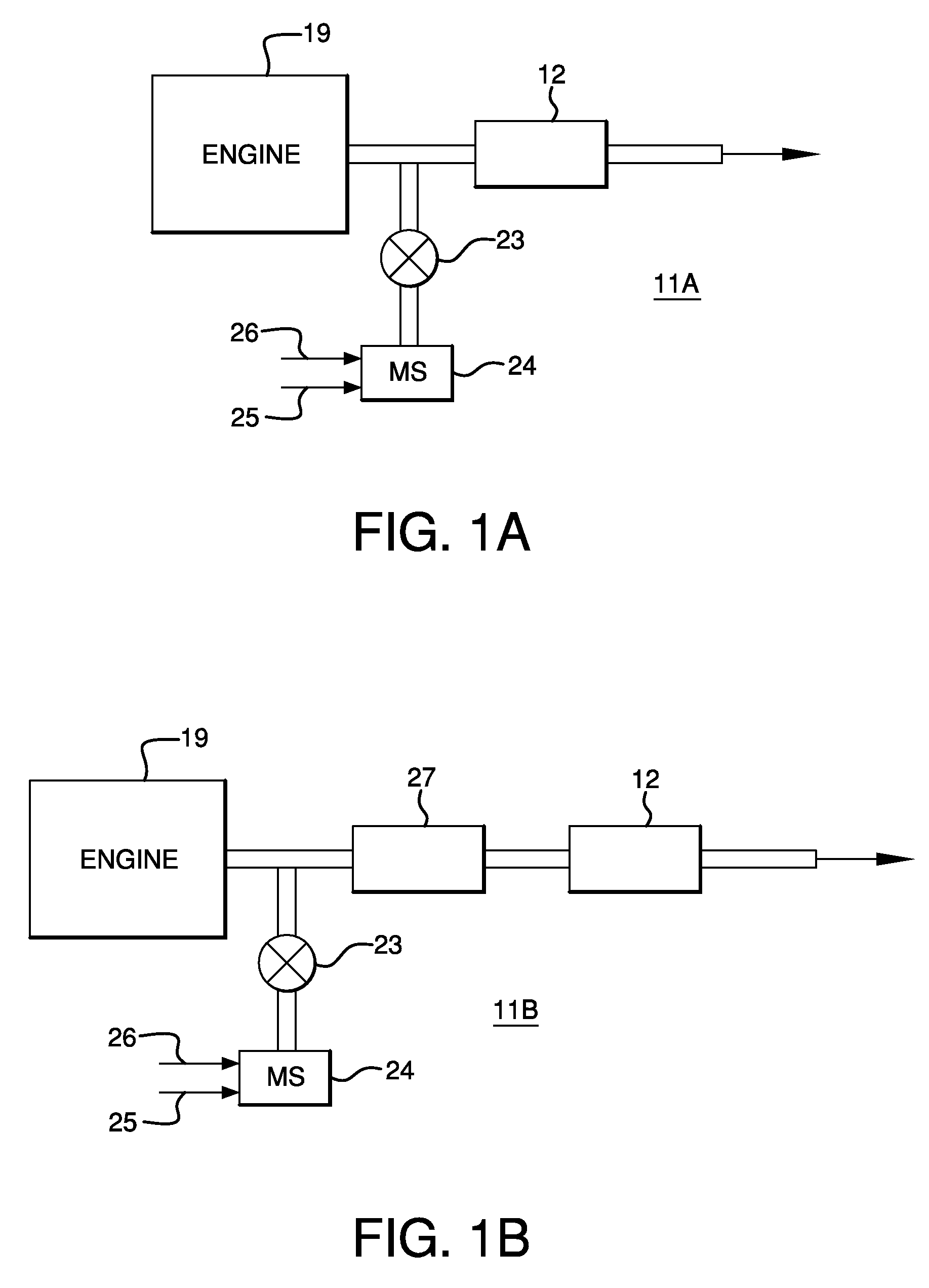
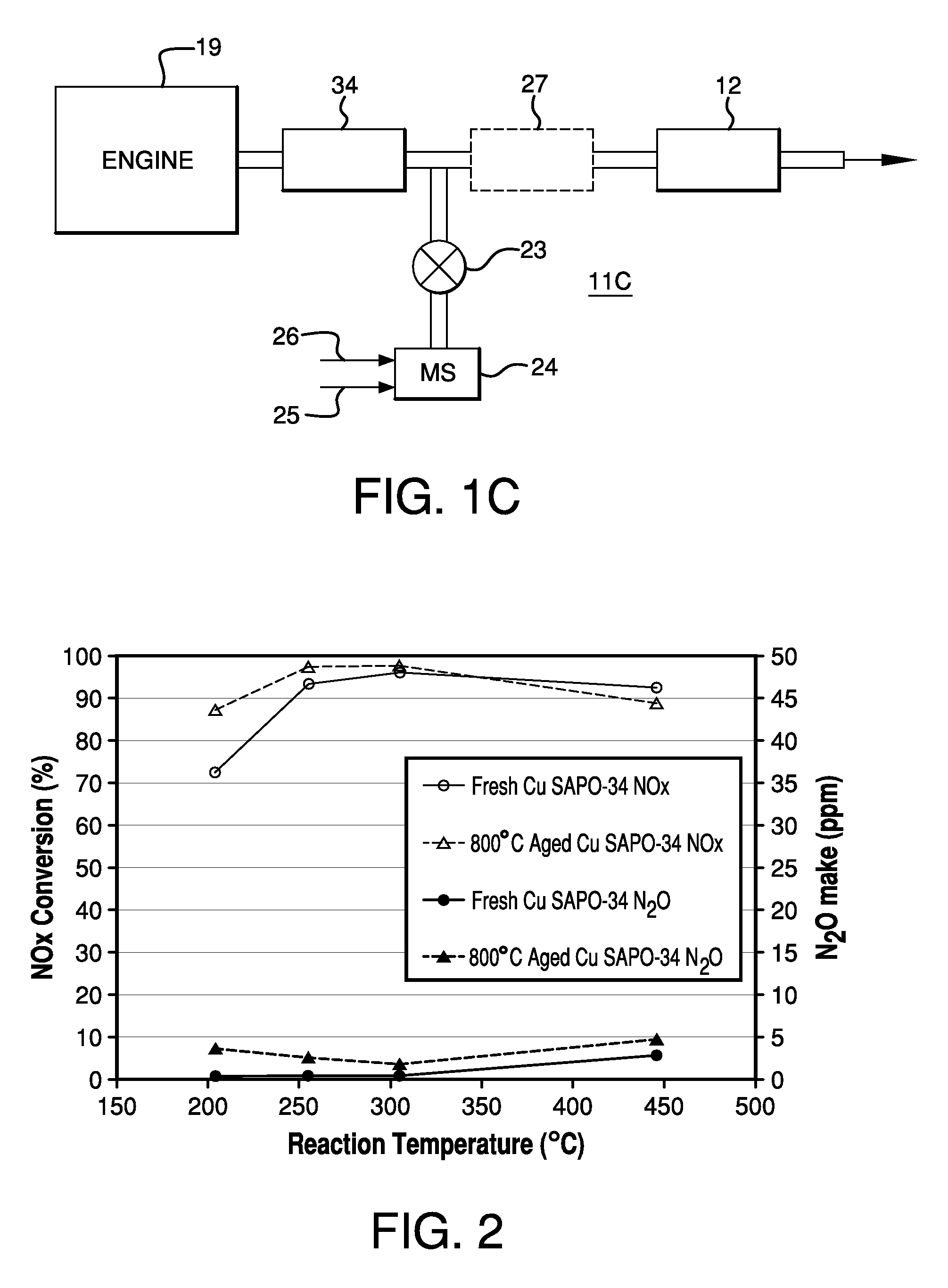
Catalysts, Systems and Methods Utilizing Non-Zeolitic Metal-Containing Molecular Sieves Having the CHA Crystal Structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0078]A SAPO-34 was prepared from a gel composition of 1.0 Al2O3:1.06 P2O5:1.08 SiO2:2.09 R:66H2O. A reaction mixture was formed by combining 1.54 kg of 85 wt. % orthophosphoric acid with a mixture of 920 g of a pseudoboehmite alumina (Catapal B) in 1.8 kg of deionized water. Then a further 1 kg of deionized water was added. The mixture was stirred until homogeneous. To this mixture was added a mixture containing 409 g of fumed silica (Aerosil-200), 1.16 kg morpholine (Aldrich, 99%) and 1.5 kg deionized water. The silica-containing mixture was added slowly with stirring and stirred until homogeneous. A further 2.5 kg of deionized water was added and the mixture was stirred until homogenous. The resulting gel was transferred to 5 gallon autoclave where it was aged at 38° C. for 24 hours. This was then heated in the autoclave for 24 hours at 200° C. The crystalline product was recovered via filtration and was washed to a conductivity lower than 200 μScm−1. Washing the crystalline prod...
example 2
[0087]The NH4+-form of SAPO-34 was formed using the same hydrothermal synthesis and ammonium exchange conditions detailed in example 1.
[0088]A Cu-SAPO34 powder catalyst was prepared by mixing 320 g of NH4+-form SAPO-34 with 1.28 L of a copper(II) acetate monohydrate solution of 0.5 M. The pH was between 4.0 and 4.3 during the reaction. An ion-exchange reaction between the NH4+-form SAPO-34 and the copper ions was carried out by agitating the slurry at 70° C. for 1 hour. The resulting mixture was then filtered, washed until the filtrate had a conductivity lower than 200 μScm−1, which indicated that substantially no soluble or free copper remained in the sample, and the washed sample was dried at 90° C. The obtained Cu-SAPO34 catalyst comprised CuO at 3.18% by weight. The BET surface area of this sample as prepared was 307 m2 / g. The BET surface area of this sample after aging at 850° C. in 10% steam for 6 hours was 303 m2 / g.
[0089]The slurry preparation, coating and SCR NOx evaluation ...
example 3
[0090]A SAPO-44 was prepared from a gel composition of 1.0 Al2O3:1.0 P2O5:1.0 SiO2:1.9 R:63 H2O. A reaction mixture was formed by combining 1.54 kg of 85 wt. % orthophosphoric acid with a mixture of 971 g of a pseudoboehmite alumina (Catapal B) in 4 kg of deionized water. The mixture was stirred until homogeneous. To this mixture was added a mixture containing 399.6 g of fumed silica (Aerosil-200), 1.25 kg cyclohexylamine (Aldrich, 99%) and 2.66 kg deionized water. The silica-containing mixture was added slowly with stirring and stirred until homogeneous. The resulting gel was transferred to 5 gallon autoclave where it was heated in the autoclave for 48 hours at 190° C. The crystalline product was recovered via filtration and was washed to a conductivity lower than 200 μScm−1. The sample was dried before calcining at 600° C. for 4 hours. The crystalline product had an X-ray powder diffraction pattern indicating that it was SAPO-44, a non-zeolitic molecular sieve with the chabazite t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com