Treatment and prevention of viral infections
a technology for viral infections and treatment, applied in the field of treatment and prevention of viral infections, can solve the problems of ineffective current treatment of chronic infection, unknown exact mechanism of viral entry, and high risk of infection, and achieve the effect of reducing or eliminating the immunogenicity of antibodies and maintaining the specificity of mouse antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0196]The isolation of cDNA sequences encoding E1E2 glycoproteins from patients infected with different genotypes of HCV was reported by Lavillette et al., (43). To generate HCV pseudoparticles (HCVpps) enveloped with glycoproteins derived from different genotypes, the inventors used vectors expressing appropriate HCV E1E2 and murine leukaemia virus (MLV) Gag-Pol. The inventors also utilised a MLV transfer vector encoding the GFP reporter protein to act as a transduction marker.
[0197]The plasmids expressing the HCV genotype 1a strain H-derived full-length E1E2, murine leukaemia virus (MLV) Gag-Pol, and the MLV transfer vector carrying GFP under the control of human CMV promoter have been described previously (6). The cDNA sequences encoding the full-length E1E2 of HCV from various clinical isolates [representing amino acid residues 170 to 746 of the HCV open reading frame referenced to strain H77c (70)] were generated by PCR, cloned downstream from a human CMV promoter in the expres...
example 2
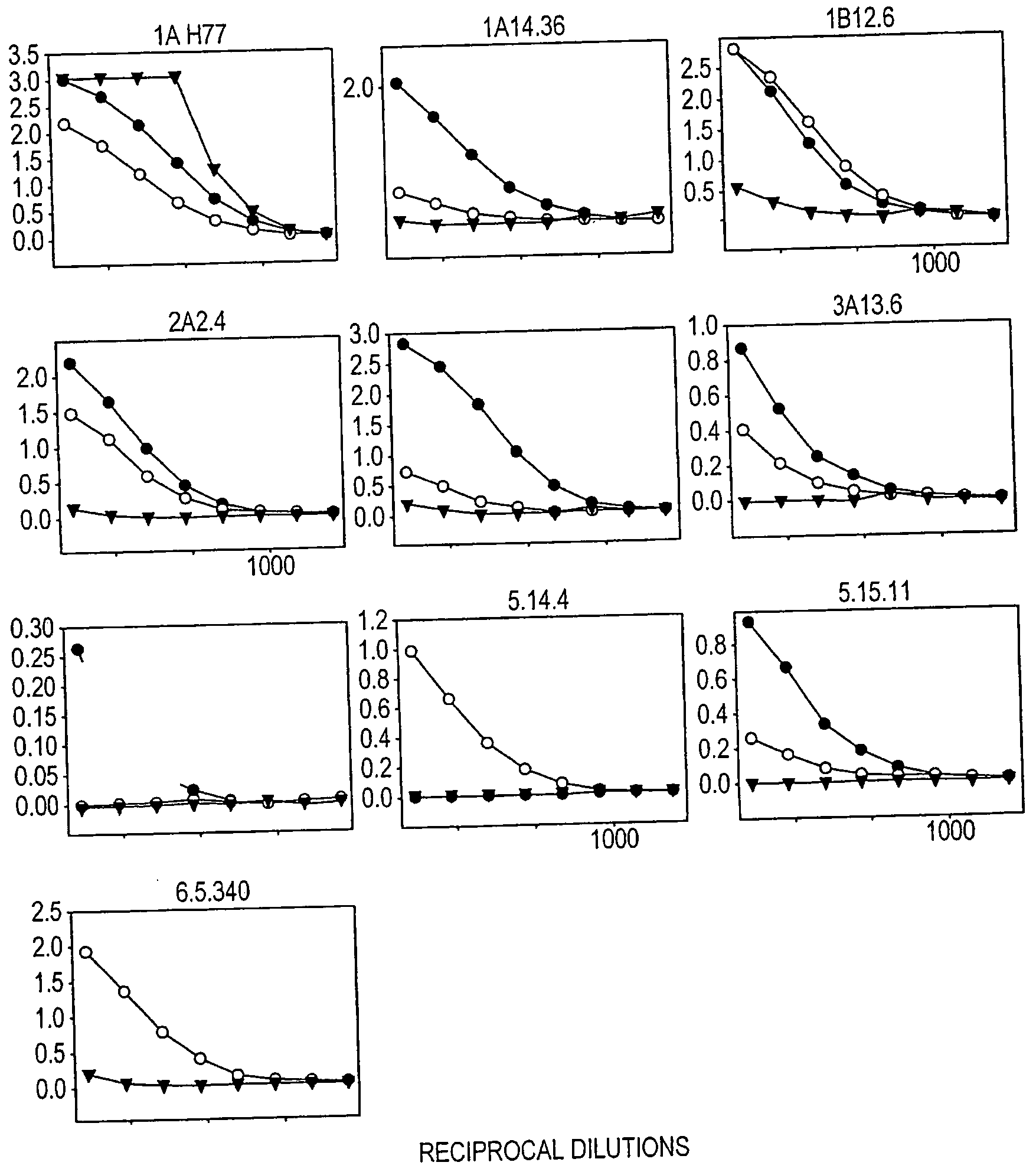
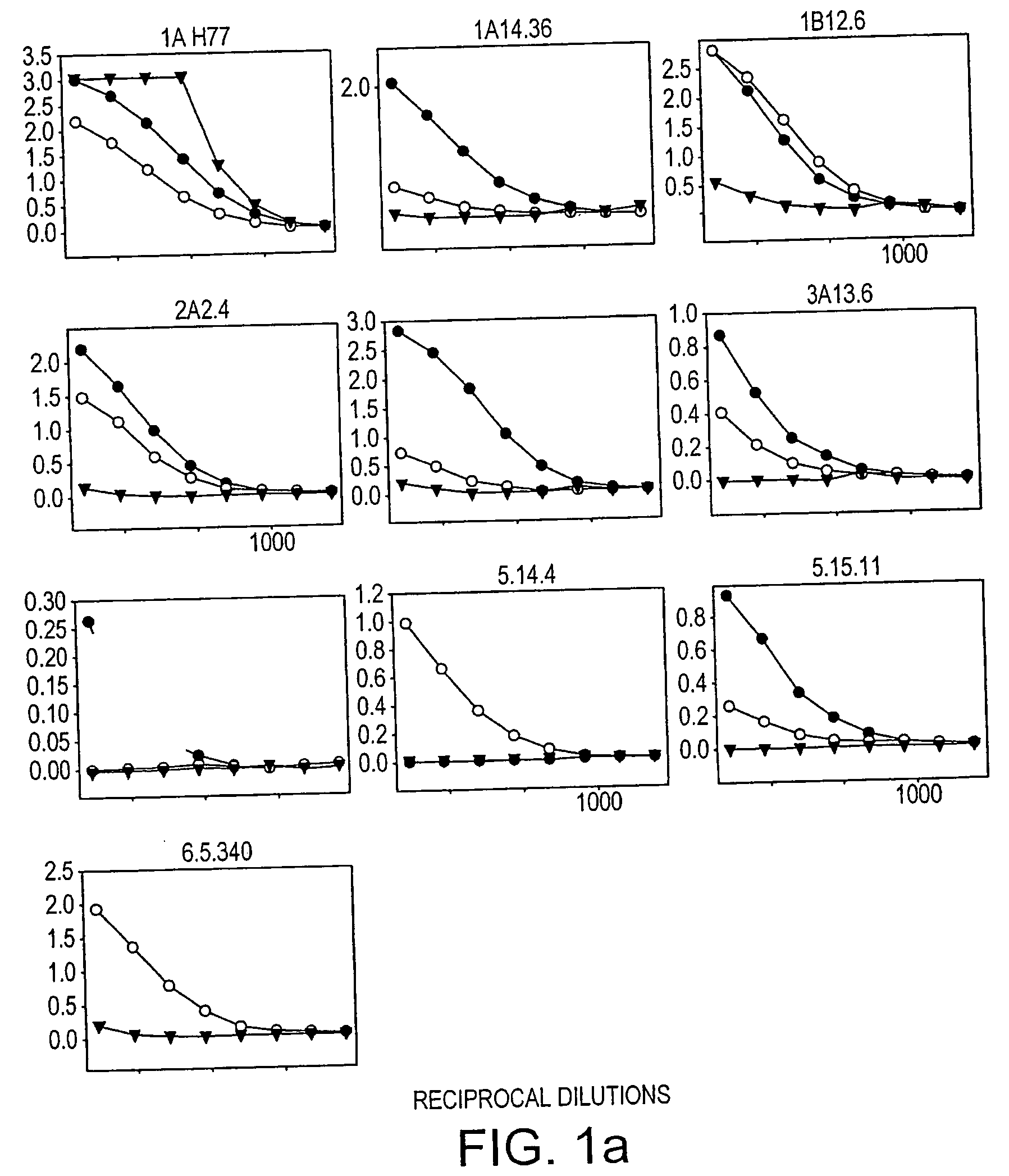
[0201]To investigate why many of the isolates lacked infectivity, the inventors checked whether HCV glycoproteins were expressed in the transfected HEK293T cells. The relative level of the E2 glycoprotein in each cell lysate was determined by means of an ELISA involving GNA (Galanthus nivalis) lectin-coated ELISA plates (Dynex Labsystems), and polyclonal rabbit serum R646, and two monoclonal antibodies (MAbs), AP33 and ALP98, all raised against type 1a E2 and described previously 52, 16). MAb AP33 and R646 antiserum were purified on a protein G column according to the manufacturer's protocol (Amersham Biosciences).
[0202]The ELISA assay to detect E2 glycoprotein was performed essentially as described previously (54). Briefly, the E1E2 glycoproteins from the clarified lysates of HEK293T cells co-transfected as described above were serially diluted threefold and captured on to GNA lectin-coated ELISA plates. The bound glycoproteins were detected using anti-E2 MAbs AP33 or ALP98 or rabb...
example 3
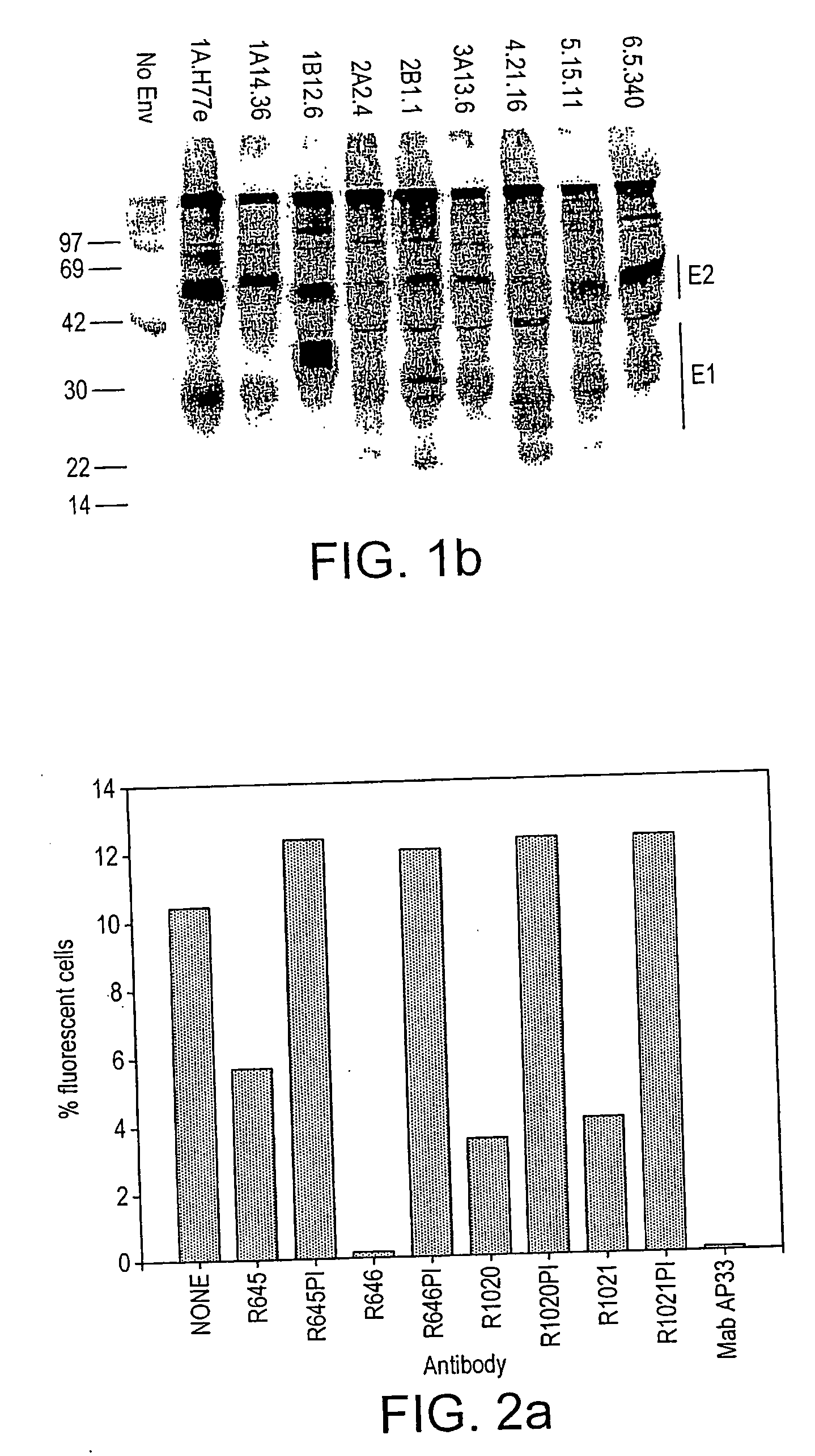
[0206]The HCVpp infectivity is dependent on the incorporation of the full-length E1E2 complex into the envelope of the particles (6, 39). Although the ELISA data above confirmed the presence of E2 derived from different genotypes, presence of E1 could not be analysed due to the lack of a broadly reactive anti-E1 antibody. Instead, the inventors investigated E1E2 complex formation by immunoprecipitation assay. HEK293T cells co-transfected with the HCV glycoprotein-expressing constructs and the MLV Gag-Pol and GFP transfer vector were radiolabelled with [35S]methionine / cysteine.
[0207]Radiolabelling was performed as follows.
[0208]Eighteen hours following transfection, cells were washed with PBS, and incubated in methionine / cysteine-free medium containing 25 μCi / ml of L-[35S] Redivue™ Pro-Mix™ (Amersham Biosciences) for 48 h. The medium of transfected cells was harvested and clarified by centrifugation. The cells were washed with PBS, lysed in lysis buffer (20 mM Tris-HCl, pH 7.4, 20 nM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com