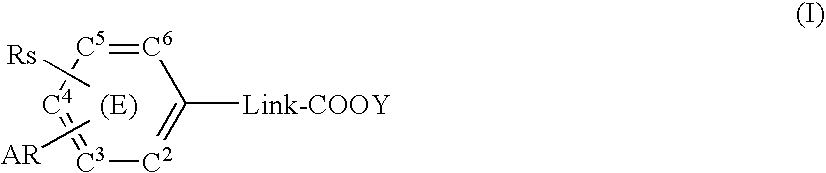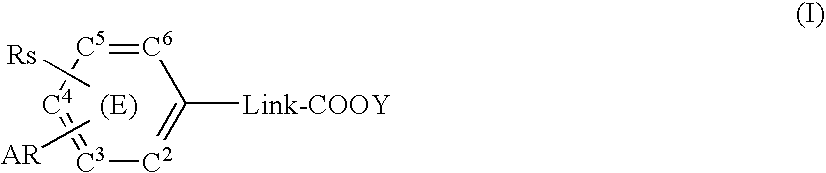Substituted arylalkanoic acid derivatives and use thereof
a technology of arylalkanoic acid and derivatives, which is applied in the field of substituted arylalkanoic acid derivatives, can solve the problems of increased production of leukotrienes, side effects, and small difference between effective dose and dose inducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0621]The present invention will be further specifically explained with reference to examples. However, the scope of the present invention is not limited to the following examples. In the examples, for thin layer chromatography (TLC), Precoated Silica Gel 60 F254 (produced by Merck, product number: 5715-1M)) was used. After development with chloroform:methanol (1:0 to 1:1), acetonitrile:acetic acid:water (200:1:1 to 100:4:4) or ethyl acetate:hexane (1:0 to 0:1), spots were observed by UV irradiation (254 nm) or color development with ninhydrine or dinitrophenylhydrazine solution in hydrochloric acid. For drying organic solvent, anhydrous magnesium sulfate or anhydrous sodium sulfate was used. As for column chromatography, the indication of “Quad” means use of Quad 1 preparative chromatography system (produced by Biotage), and one or several columns selected from cartridge columns KP-Sil-12M, 40S and 40M produced by the same manufacturer were used depending on the amount of sample. F...
example a-1
Synthesis of methyl 3-(4-hydroxyphenyl)propionate (Intermediate 1)
[0627]A solution obtained beforehand by adding thionyl chloride (18.3 ml, WAKO) dropwise to methanol (250 ml) and mixing the mixture under ice cooling was added dropwise with a solution of 3-(4-hydroxyphenyl)propionic acid (16.6 g, TCI) in methanol (50 ml) under ice cooling, stirred for 30 minutes, warmed to room temperature, and further stirred for 1.5 hours. The reaction mixture was concentrated under reduced pressure, and then extracted with diethyl ether (200 ml). The organic layer was washed successively with saturated aqueous sodium hydrogencarbonate, saturated aqueous ammonium chloride and saturated brine. The organic layer was dried, and then the solvent was evaporated under reduced pressure to obtain the title compound (Intermediate 1, 17.95 g).
Synthesis of methyl 3-(4-cyclopentylmethyloxyphenyl)propionate (Intermediate 2)
[0628]A solution of cyclopentane methanol (4.05 ml, Ald) in anhydrous tetrahydrofuran (a...
example a-2
Synthesis of 3-(3-bromo-4-methoxyphenyl)propionic acid (Intermediate 3)
[0630]According to the procedure described in the synthesis method of Compound No. A-1 provided that the reaction was carried out under ice cooling for 30 minutes and at room temperature for 3 hours, 3-(4-methoxyphenyl)propionic acid (27.0 g, TCI) and NBS (29.4 g) were reacted and treated to obtain the title compound (Intermediate 3, 38.1 g).
Synthesis of 3-(3-bromo-4-hydroxyphenyl)propionic acid (Intermediate 4)
[0631]According to a procedure described in a literature (Carreno, M. C., J. Org. Chem., 1995, vol. 60, p. 5328), a 1 M solution of boron tribromide in methylene chloride (200 ml, Fluka) was added dropwise with a solution of Intermediate 4 (23.5 g) in methylene chloride (200 ml) at −78° C., warmed to room temperature after 30 minutes, and further stirred for 1.5 hours. The reaction mixture was poured into ice water (750 ml), and stirred at room temperature for 1 hour. The reaction mixture was added with di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com