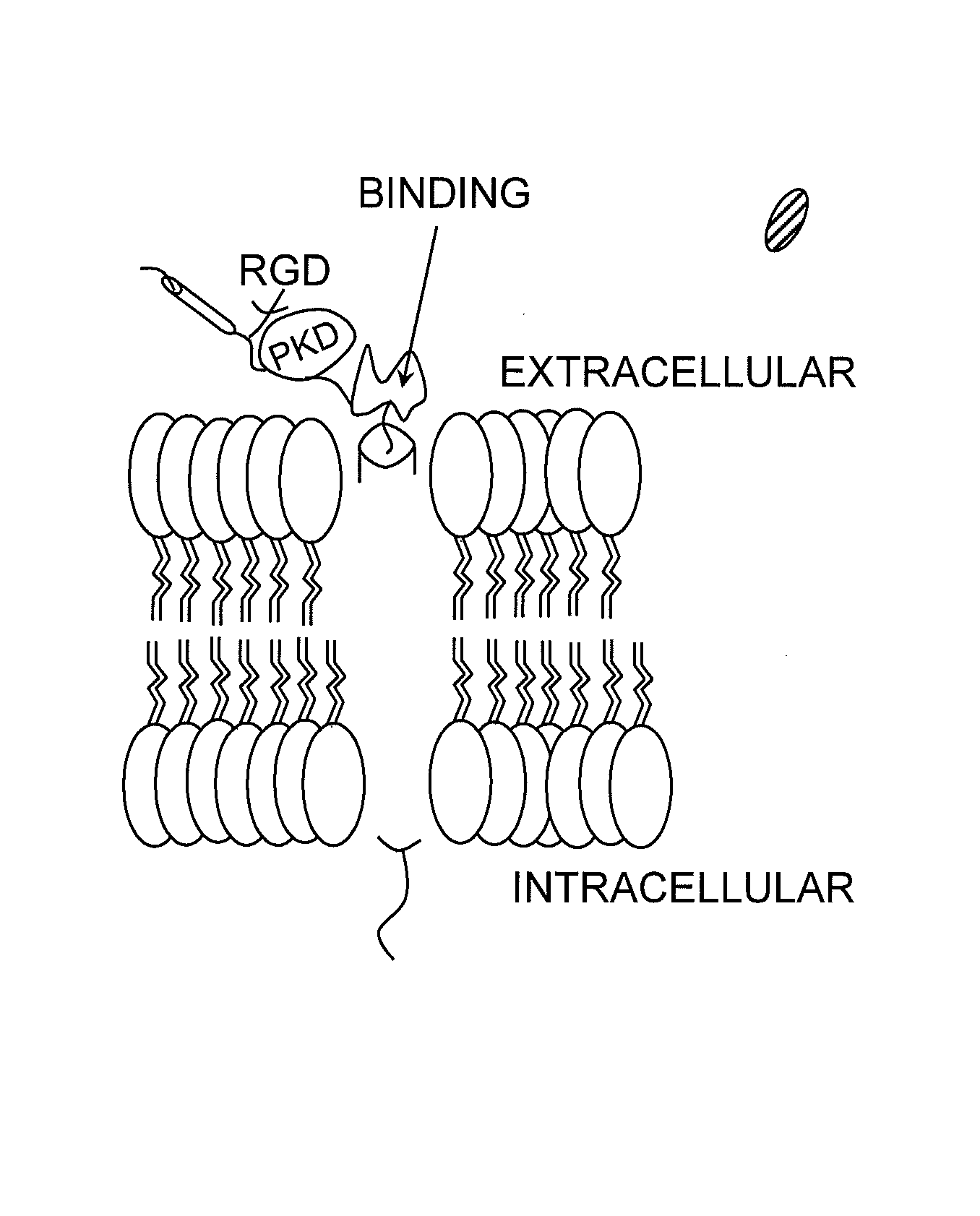
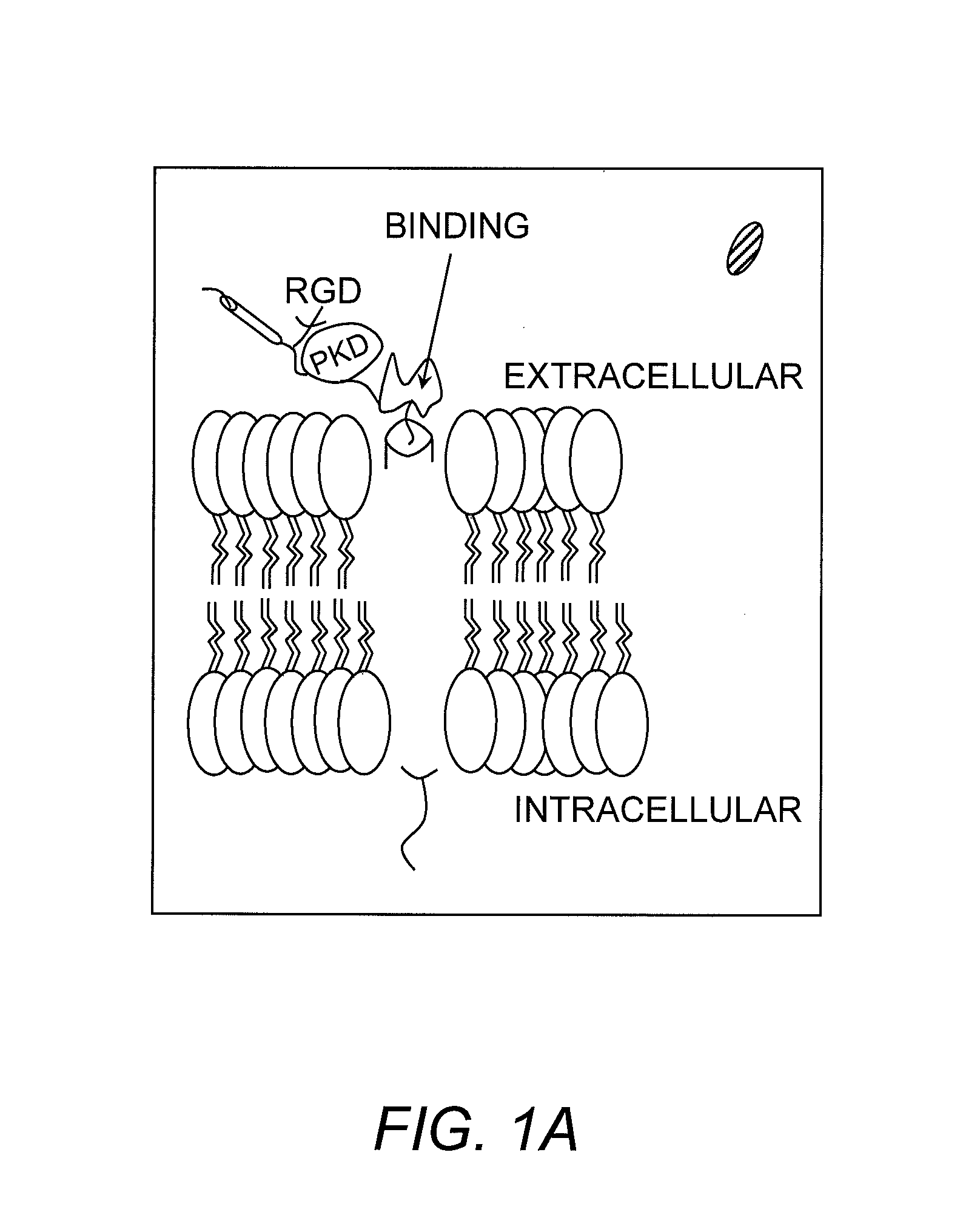
Hematopoietic growth factor inducible neurokinin-1 gene and uses thereof
a technology of hematopoietic growth factor and neurokinin, which is applied in the direction of genetic material ingredients, peptides, drug compositions, etc., can solve the problems of stem cells, bone marrow may not produce enough stem cells, breast cancer metastasis to the bone marrow, etc., to reduce the toxicity of normal tissues and increase the effectiveness of chemo- and radiotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0203] Unless otherwise specified, general cloning procedures, such as those set forth in Sambrook et al., Molecular Cloning, supra or Ausubel et al. (eds) Current Protocols in Molecular Biology, John Wiley & Sons (2000) were used herein.
[0204] Reagents. Hoffman-La Roche (Nutley, N.J.) provided recombinant human (rh)IL-1α. Stem cell factor (rhSCF), rhIL-6, rhIL-11 and alkaline phosphatase (Alk Phos)-conjugated goat anti-rabbit IgG were purchased from R&D Systems (Minneapolis, Minn.). IL-1β and nerve growth factor (NGF) were purchased from Collaborative Research (Bedford, Mass.) and Amersham Life Science (Cleveland, Ohio) respectively. The following was purchased from Sigma (St Louis, Mo.): lsopropyl-D-Thioglactopyranoside (IPTG), SP, FICOLL HYPAQUE, lipopolysaccharide (LPS), Fibronectin-Fragment III-C (FN-IIIC), 12-0-tetradecanoylphorbol diester (TPA), dimethylsulfoxide (DMSO) and cytochemical staining kits for 2-naphthyl-acetate esterase and naphthol AS-D chl...
example 2
Purification of HGFIN from a Prokaryotic Expression Vector
[0224] PCR was used to amplify the coding region of HGFIN, +60 / +1760 (GENBANK accession number AF322909). The following primer pairs were used in the PCR reaction: 5′-CGG GGT ACC ATG GAA TGT CTC TAC TA-3′ (SEQ ID NO:17) (upstream with KpnI linker) and 5′-CCG GAA TTC TCG AAA TTT AAG AAA CT-3′ (SEQ ID NO:18) (downstream with EcoRI linker). The HGFIN-specific sequences are underlined for both the upstream and downstream primers.
[0225] The amplified DNA fragment was cloned into pHAT10, hereafter referred as pHAT10-HGFIN. The vector was transformed into bacteria and HGFIN-HAT induced with IPTG. Induced bacterial cultures (20 ml) were sonicated in 2 ml of 100 mM Tris, pH 6.8, 4% SDS. After this, HGFIN was verified in the cell-free lysates by western blot analysis using 15 μg of total protein and rabbit anti-HAT. Details on the technique for western blot are described herein. The lysates that showed a band at the predicted size of...
example 3
ProteinChip Analyses for HGFIN-SP Interaction
[0226] Before studying the interaction between SP and HGFIN, each protein was profiled by the Surface Enhanced Laser Desorption / Ionization (SELDI) ProteinChip Array technology (Ciphergen Biosystems Inc., Fremont, Calif.). Normal phase (NP1) arrays were used for profiling and preactivated surface arrays (PSi) for HGFIN-SP interaction. For profiling studies, 2 μg of purified HGFIN or 2 μg of SP were spotted directly onto the NP1 arrays. Prior to adding of the proteins, chips were pre-wet with PBS. Arrays were incubated at room temperature until the protein was absorbed, which took approximately 5 to 10 minutes. After this, 0.5 μl of sinapinic acid (SPA) (Ciphergen Biosystems), diluted at 1:50 in 50% acetonitrile and 0.5% trifluoroacetic acid was added to the arrays. Chips were immediately analyzed using linear, time-lag focusing laser desorption / ionization SELDI-time-of-flight mass spectrometer (Model PBS II). Accurate mass was determined ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com