Optimized Liquid-Phase Oxidation
a technology of aromatic compound and liquid phase, which is applied in the preparation of carboxylic compounds, organic chemistry, chemistry apparatus and processes, etc., can solve the problems of unfavorable cstrs, unfavorable cstrs, and high capital costs of cstrs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0283] In this Example, certain properties of the novel CTA particles (illustrated in FIGS. 32A and 32B) and the comparative CTA (shown in FIGS. 33A and 33B) were evaluated. In particular, the CTA particles were tested for particle size, BET surface area, and dissolution rate. Testing of the CTA particles was performed in accordance with the test descriptions provided above in the Detailed Description section of this document.
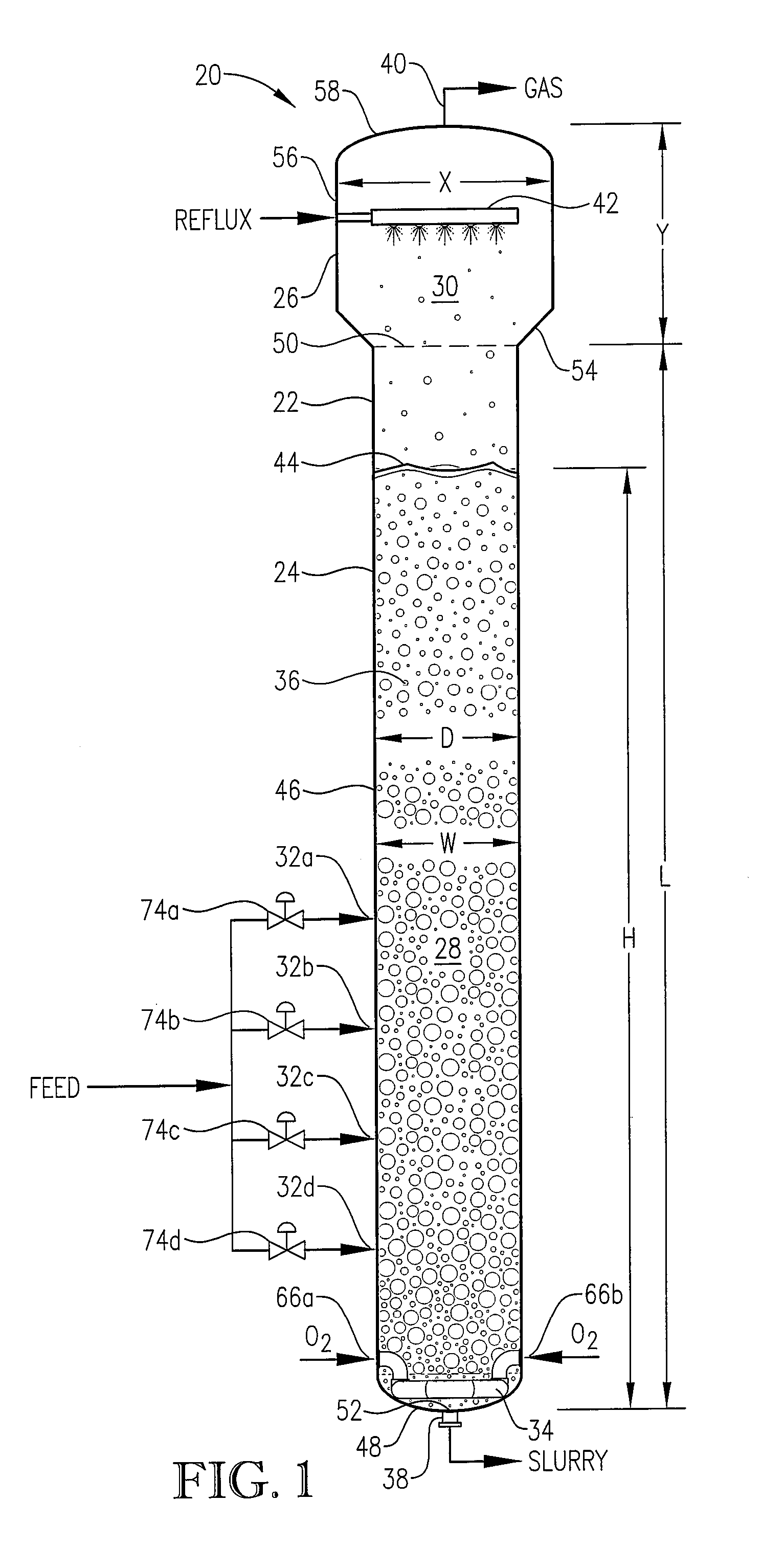
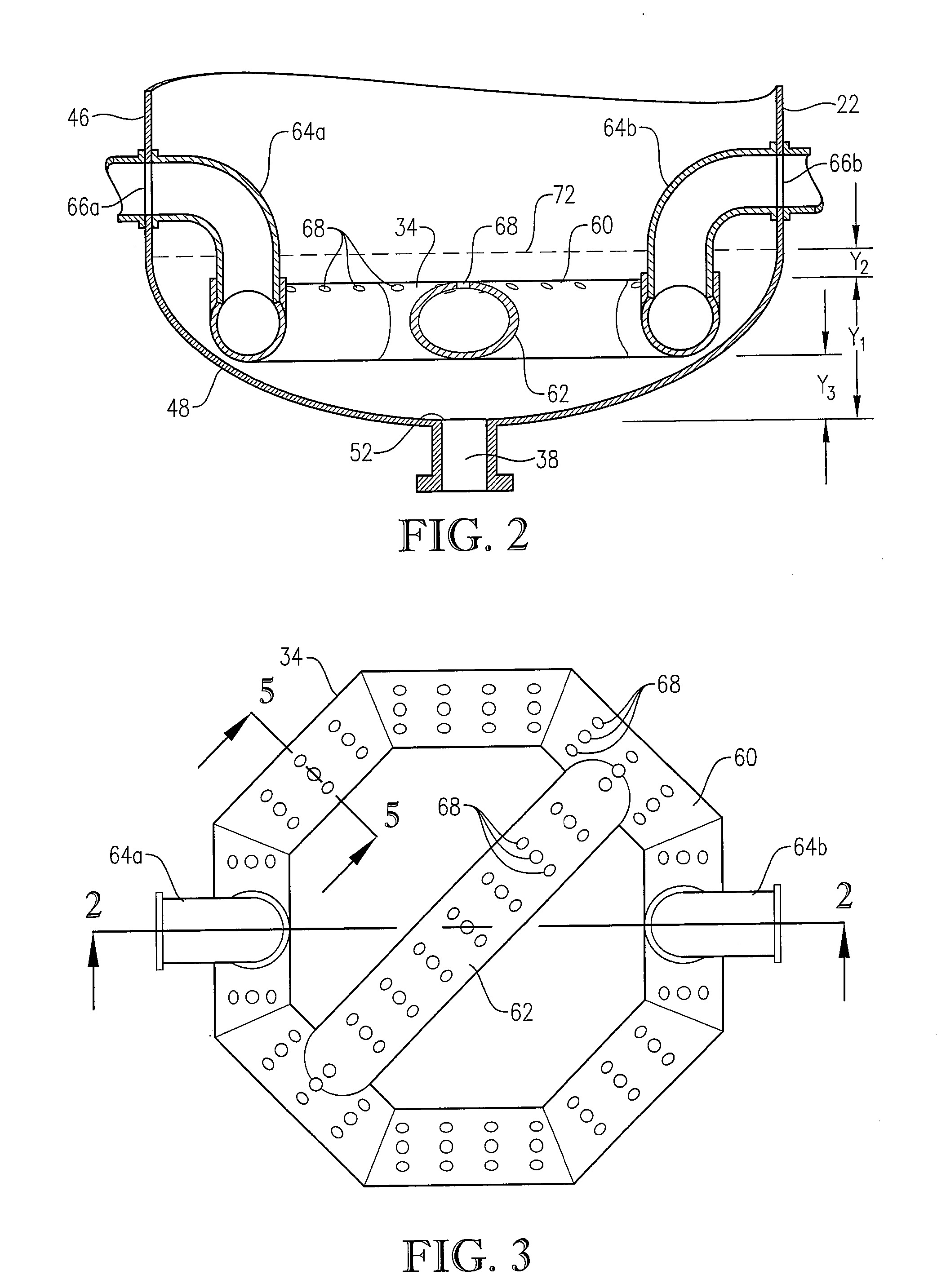
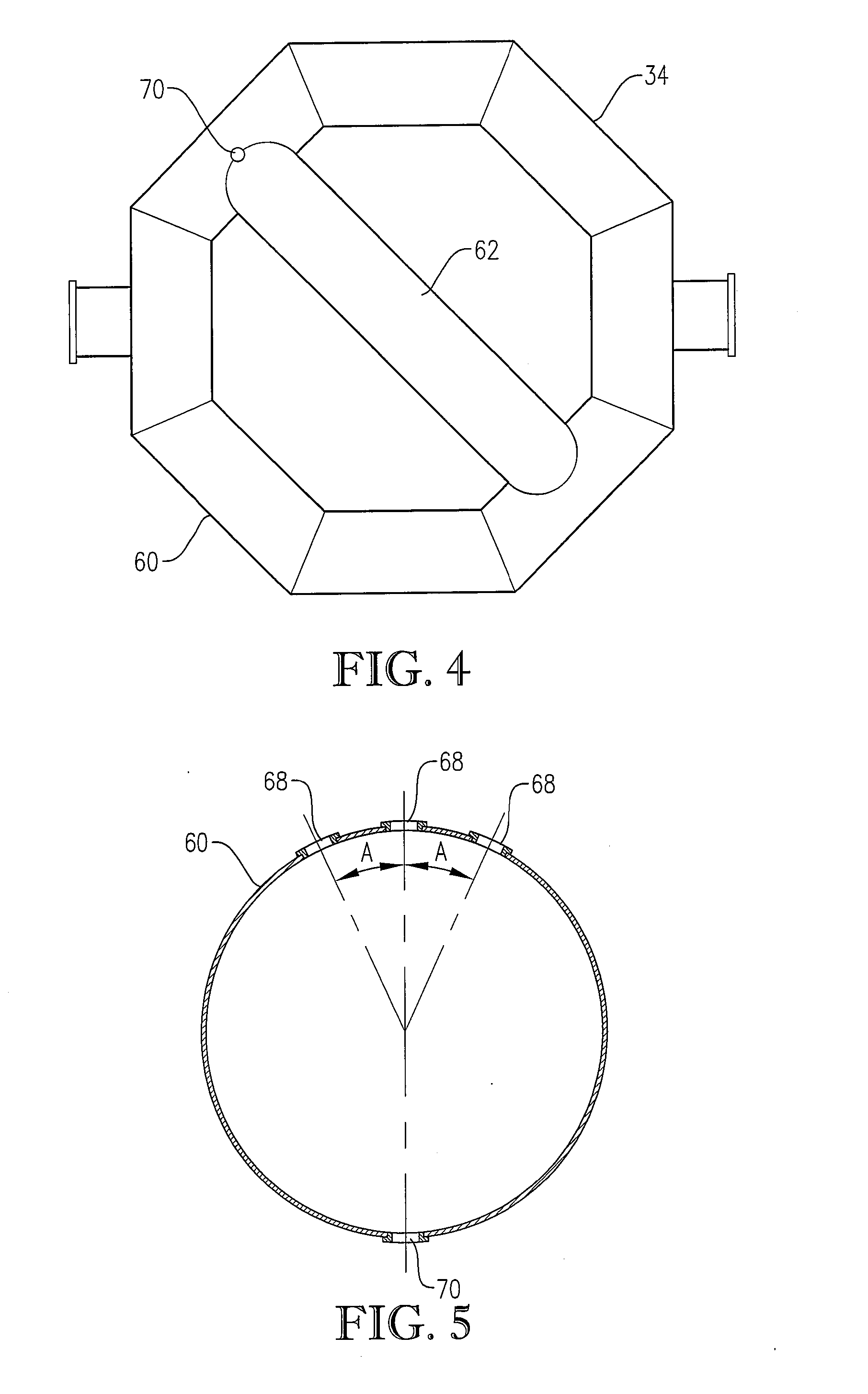
[0284] The novel CTA particles were produced by partial oxidation of commercial-purity para-xylene using recycled filtrate in a bubble column reactor operated according to many embodiments of the current invention, including solvent comprising acetic acid and water, catalyst components comprising cobalt, manganese and bromine, temperature near 160° C. at the mid-elevation of the reaction medium, preferred gradients of the gas phase composition, liquid phase composition, and temperature of the reaction medium, preferred overall STR, and preferred gradients in o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com