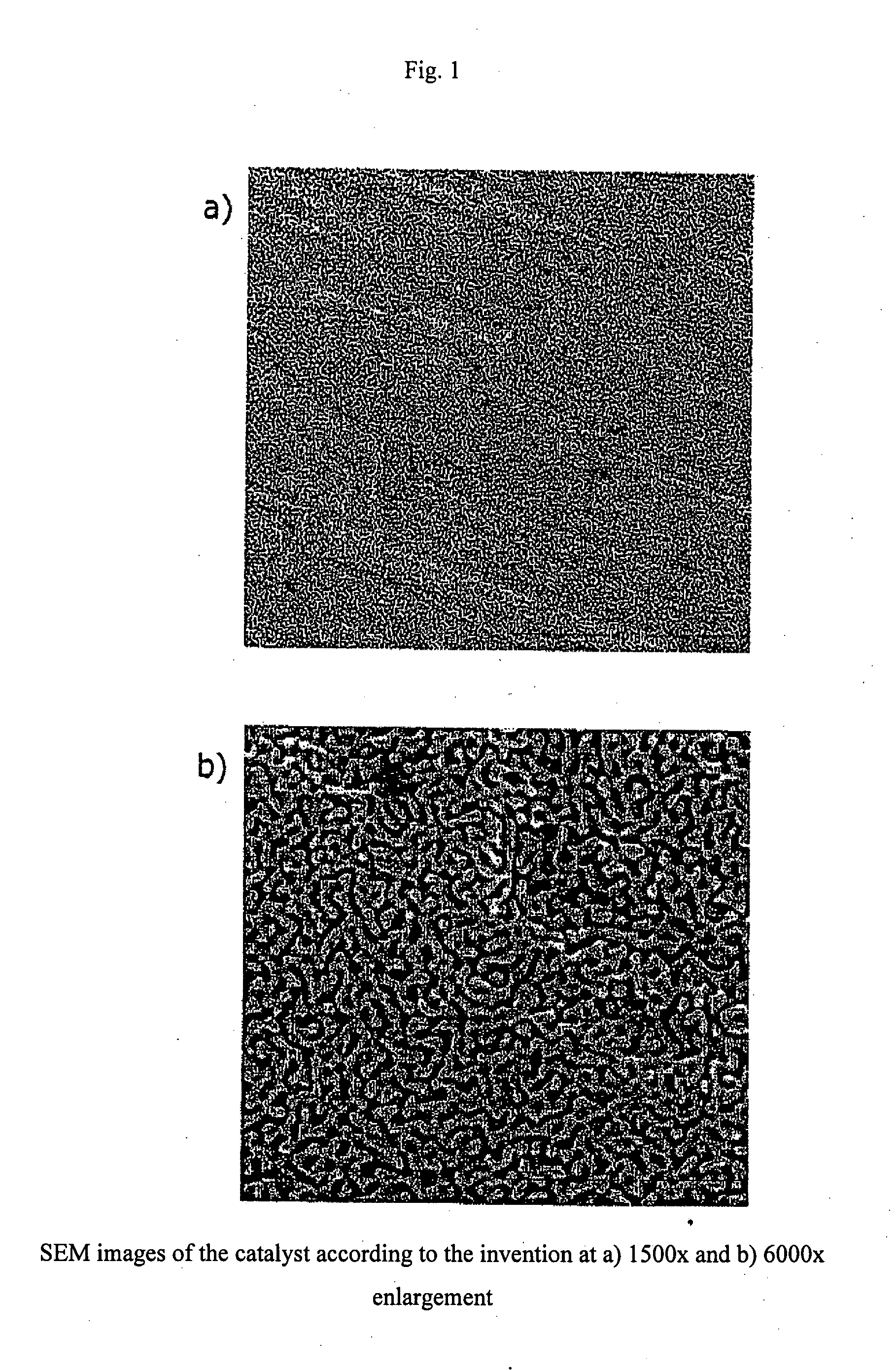
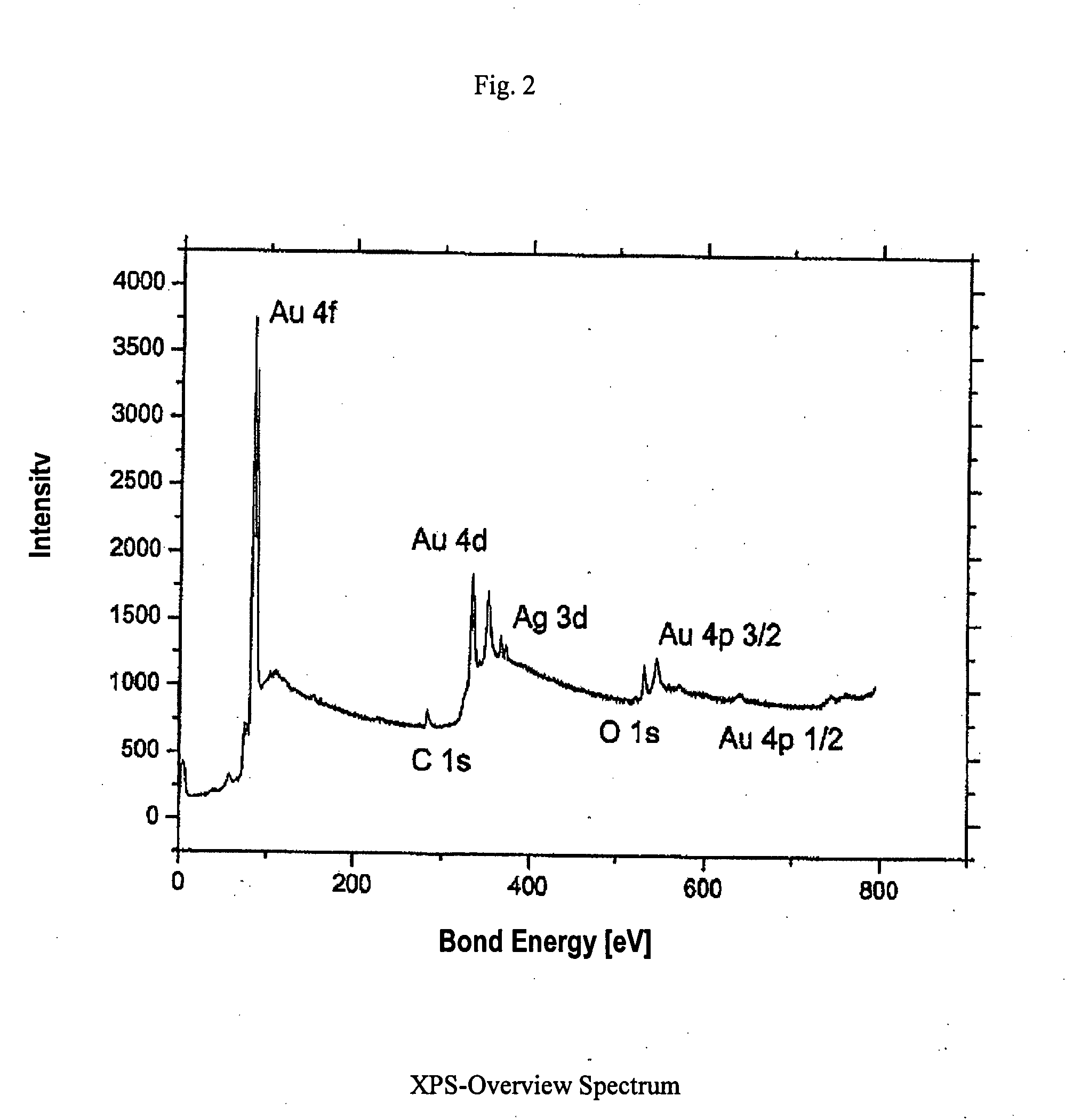
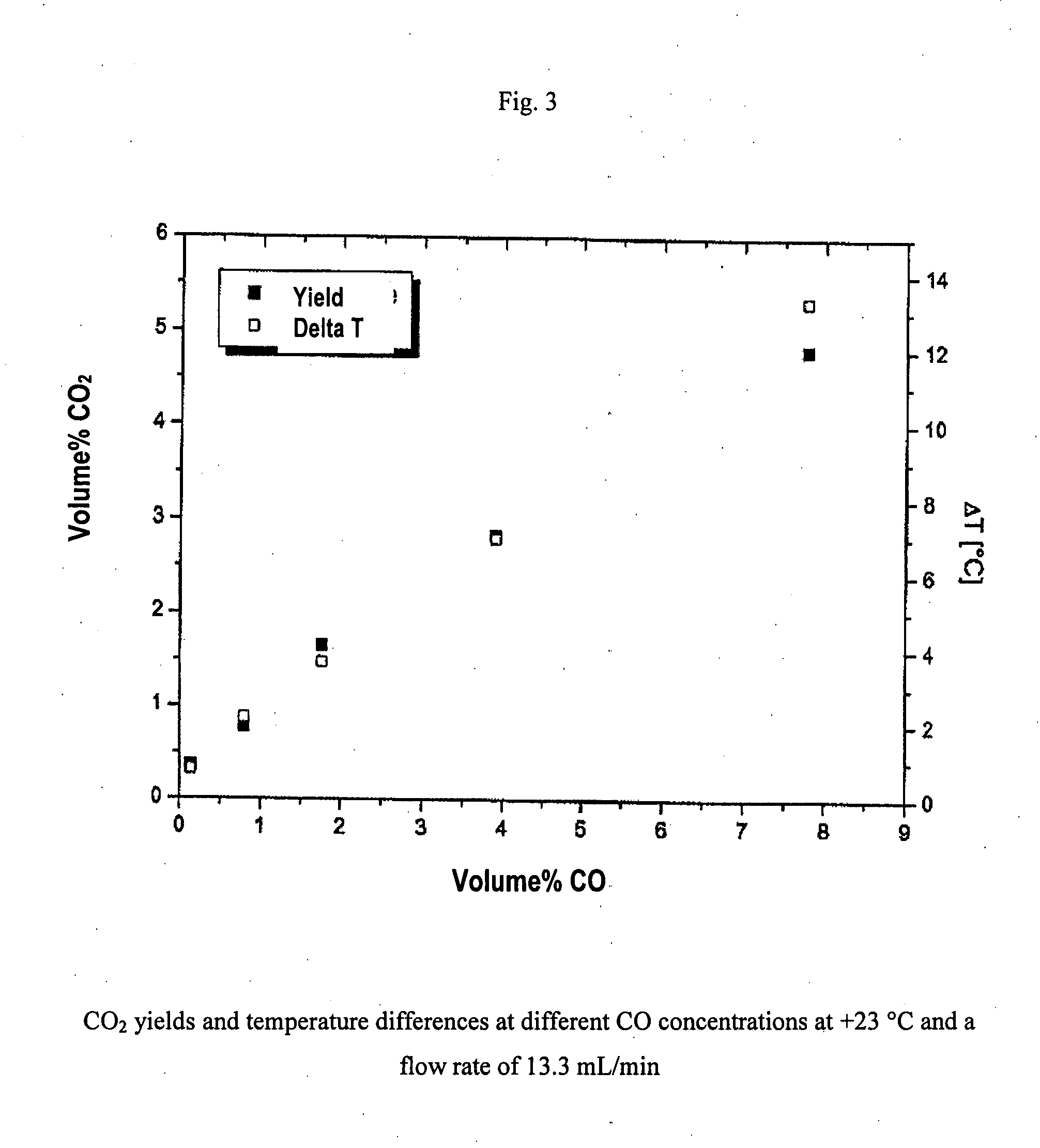
Gold-containing catalyst with porous structure
a porous structure, gold-containing technology, applied in the direction of physical/chemical process catalysts, separation processes, cell components, etc., can solve the problems of large surface energy, affecting the objective of reaction, and greatly reducing catalytic activity, etc., to achieve the highest possible activity, the effect of extending the service life and avoiding the melting poin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0059] 1. Presentation of Exemplary Gold Foams
[0060] First, an Au—Ag-starting alloy with 30 atom % Au was produced. The finished starting alloy was rolled to roughly 5 mm diameter into small pieces of desired thickness. Then, the specimens were annealed for 24 hours at 850° C. For the partial dealloying and creation of the foam structure, the specimen underwent wet-chemical treatment. The wet chemical partial dealloying was carried out in a solution of 70% nitric acid. The specimens were placed on a porous glass plate in a beaker. Then, the acid was added such that the specimen was covered. Roughly 300-μm-thick specimens were dealloyed in this manner within from one to three days. The greater part of the silver portion was selectively dissolved out of the specimens by the nitric acid, and nanoporous gold foams remained. The acid was removed using a syringe and replaced with water. This was changed out several times in order to clean remaining acid residues from the specimens. The s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com