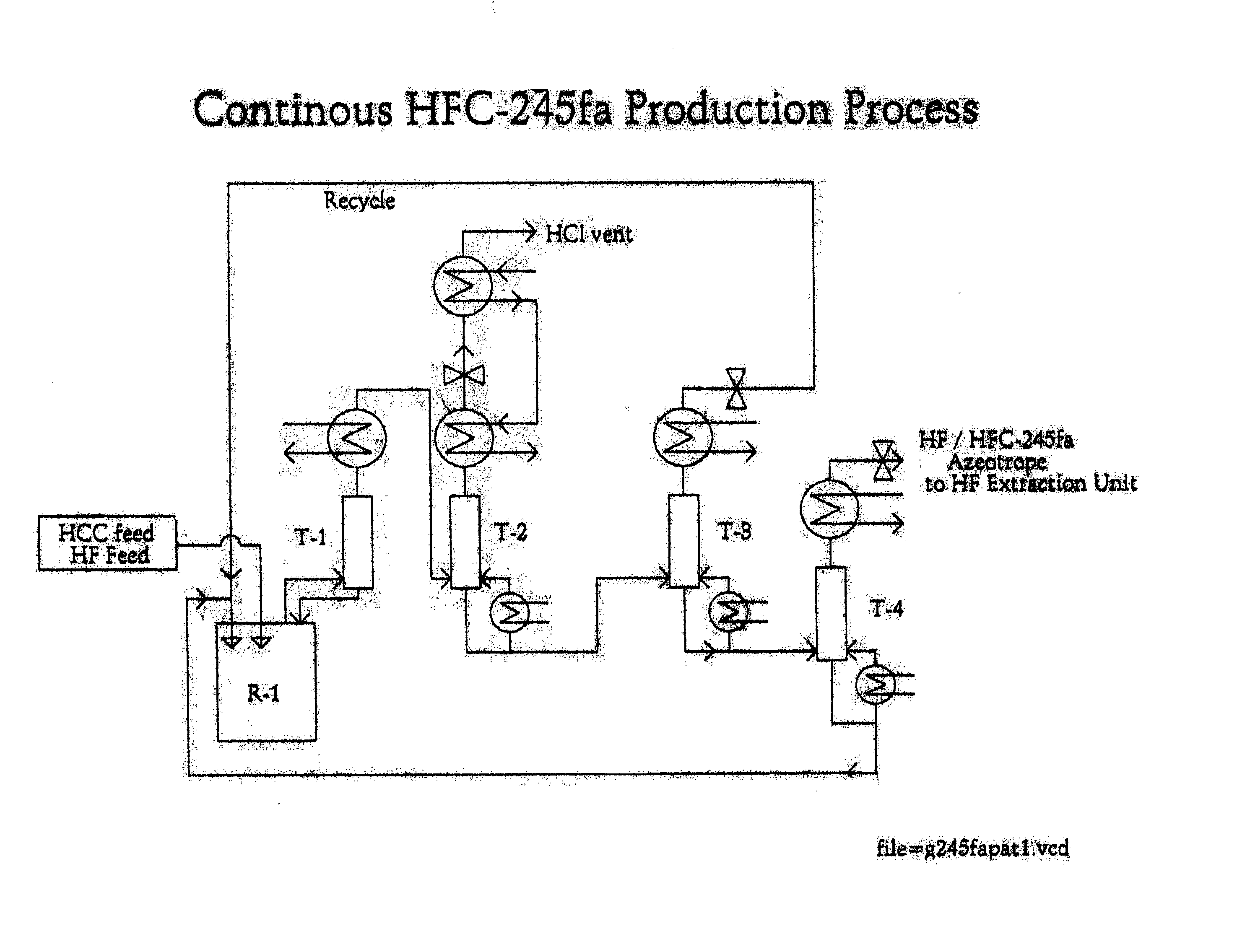
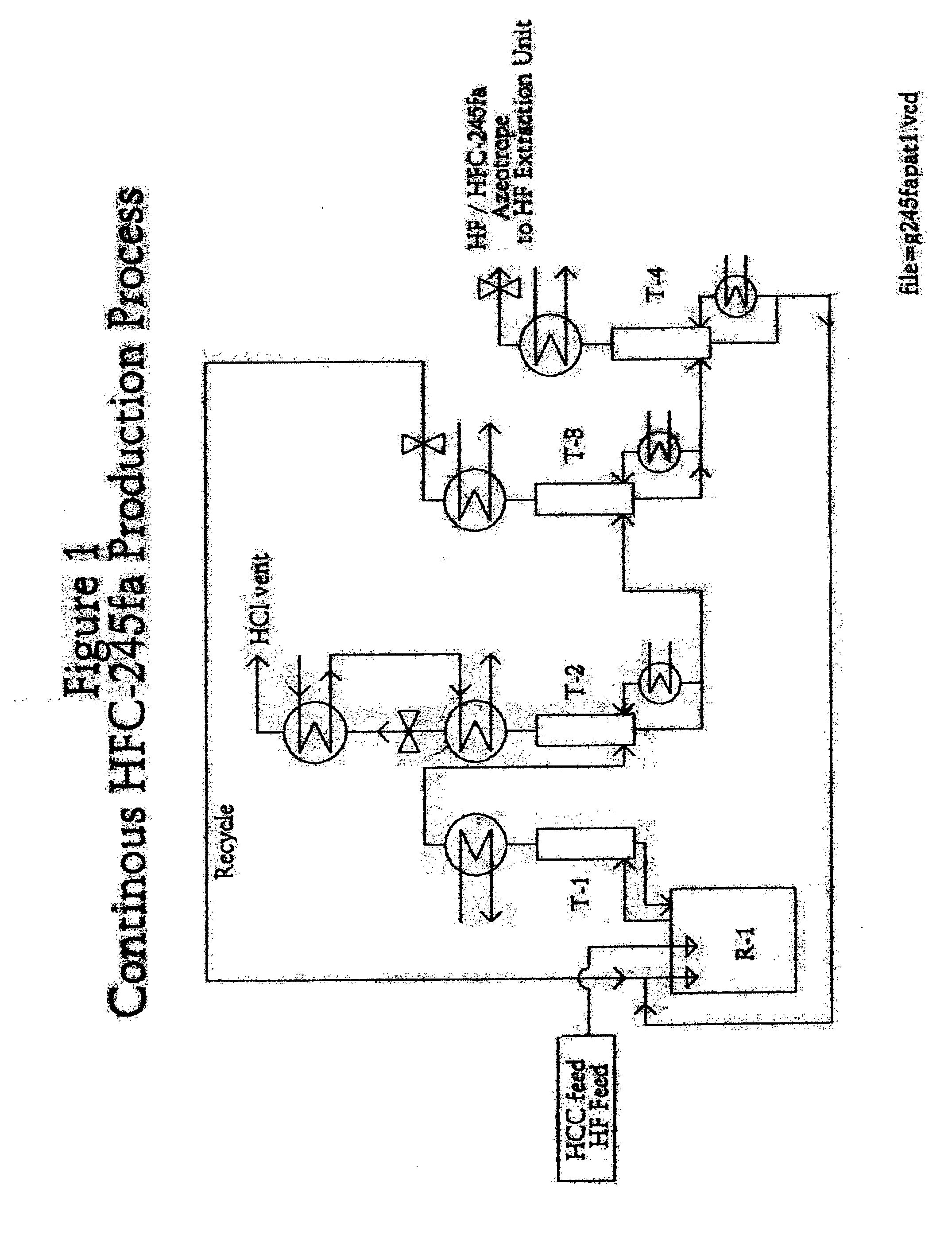
Method for preparing 1,1,1,3,3-pentafluoropropane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0040] Into a stirred 600 ml Hastelloy C autoclave was added 7.0 gram (0.032 gram-mole) of SbF5 and 56.7 grams anhydrous HF (2.84 gram-moles). Next, 85.9 grams of HFC-245fa (0.642 mole) was added, followed by 48.5 grams (0.224 mole) of HCC-240fa. The mixture was then pressurized with N2 to 170 psig, and then heated to about 120° C. over a 1 hour period and maintained at this temperature for an additional 2.5 hours. The bulk of the reaction took place in 34 minutes, as indicated by the amount of HCl byproduct vented from the system. The starting mole ratio of HF to HFC-245fa to SbF5 was 88:19.8:1. Due to the evolution of HCl, the pressure rose significantly above the HF / HFC-245fa autogeneous pressure, and gas from the autoclave at a pressure greater than 400 psig was vented through a KOH scrubber / dryer and into a liquid nitrogen chilled collection cylinder over a 34 minute period. In this acid removing scrubber, a considerable amount of the product underwent a dehydrohalogenation rea...
example 3
[0042] A corrosion study was performed on an HF / SbF5 / HFC-245fa system at 90° C. upon various metals and alloys. Using a solution of 5 wt % SbF5, 47.7 wt % HF and 47.3 wt % HFC-245fa, the corrosion rate for Hastelloy C, Inconel 600, Incoloy 825, Monel 400, SS 316 and C1018 carbon steel. The results of this example are provided in Table 1.
TABLE 1Materials of ConstructionCorrosion Rate (mils / year)Hastelloy C0Inconel 60027Incoloy 8259Monel 40030SS 31621C1018 carbon steel96
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com