Pegylated g-csf polypeptides and methods of producing same
a technology of gcsf and polypeptides, which is applied in the direction of peptide/protein ingredients, immune disorders, extracellular fluid disorders, etc., can solve the problems of insufficient uniformity of peg moieties bound to groups other than those intended, complex characterization of such proteins, and inability to meet the requirements of characterization, etc., to achieve the effect of increasing the stability and uniformity of a pegylated polypeptid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Analysis of a Partially de-PEGylated G-CSF Variant
Sample Preparation and de-PEGylation
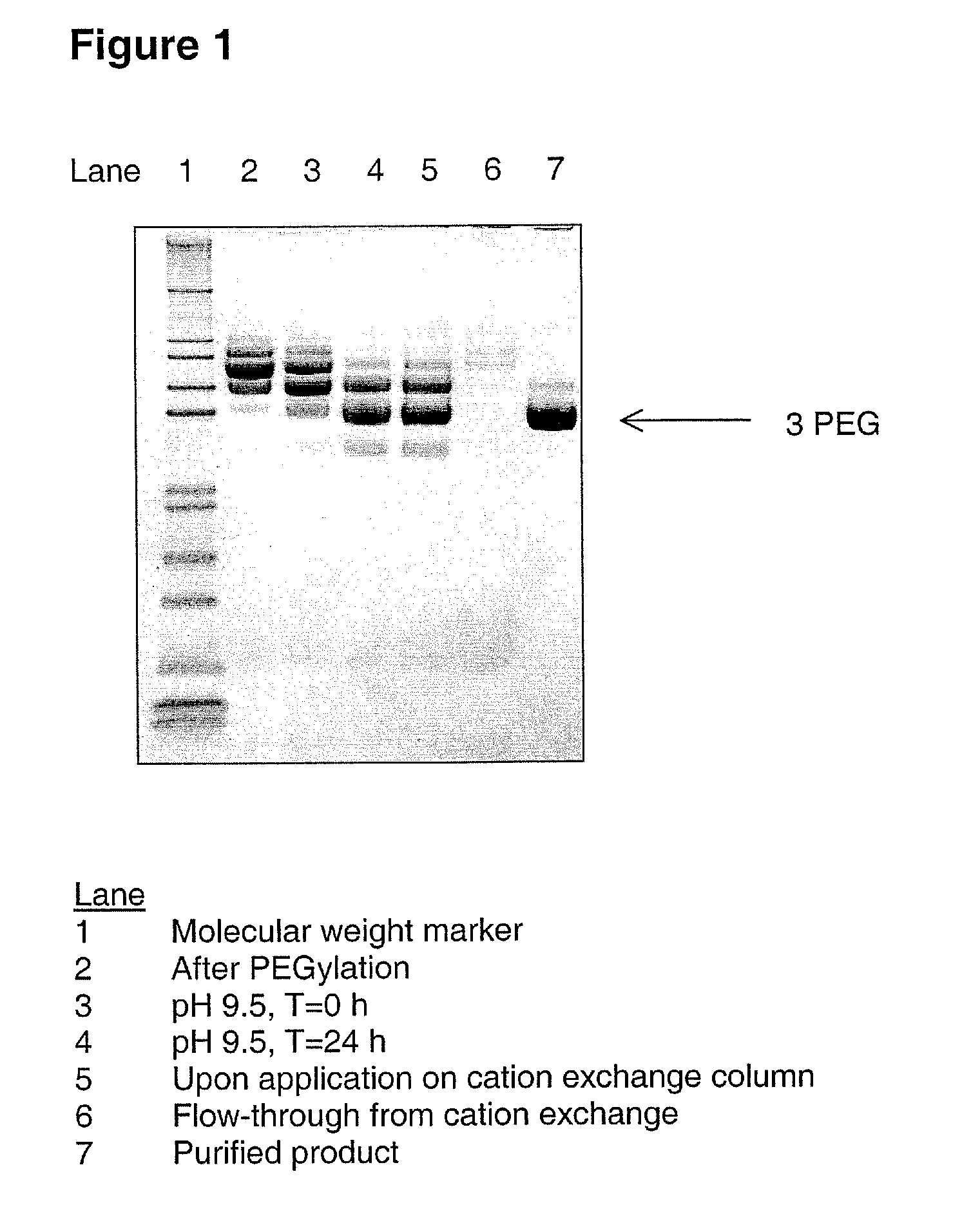
[0087] A G-CSF variant having the substitutions K16R, K34R, K40R, T105K and S159K compared to wild-type human G-CSF (SEQ ID NO:1) was produced from CHO-K1 cells substantially as described in WO 03 / 006501. 200 mL of 4.5 mg / mL of the G-CSF variant (900 mg G-CSF) was PEGylated using mPEG-SPA 5000 (Nektar Therapeutics). Briefly, 100 mL of a 13.2% (w / w) solution of mPEG-SPA 5000 was added over a period of 10 minutes to the 200 mL of G-CSF solution and gently stirred to ensure sufficient mixing. The sample was allowed to incubate for 44 minutes at 21±3° C., pH 8.5, with gentle stirring. The sample mixture was subsequently adjusted to pH 9.5 using a stock solution of 800 mM boric acid pH 10.0. The sample was then incubated at 21±3° C. for 24 hours without stirring. The sample was then diluted approx. 2.5 fold with 100 mmol / kg citric acid, 20 mmol / kg NaOH, pH 2.5, to a final pH of 3.5. ...
example 2
Preparation and Analysis of a Partially de-PEGylated G-CSF Variant
Sample Preparation, de-PEGylation and Purification
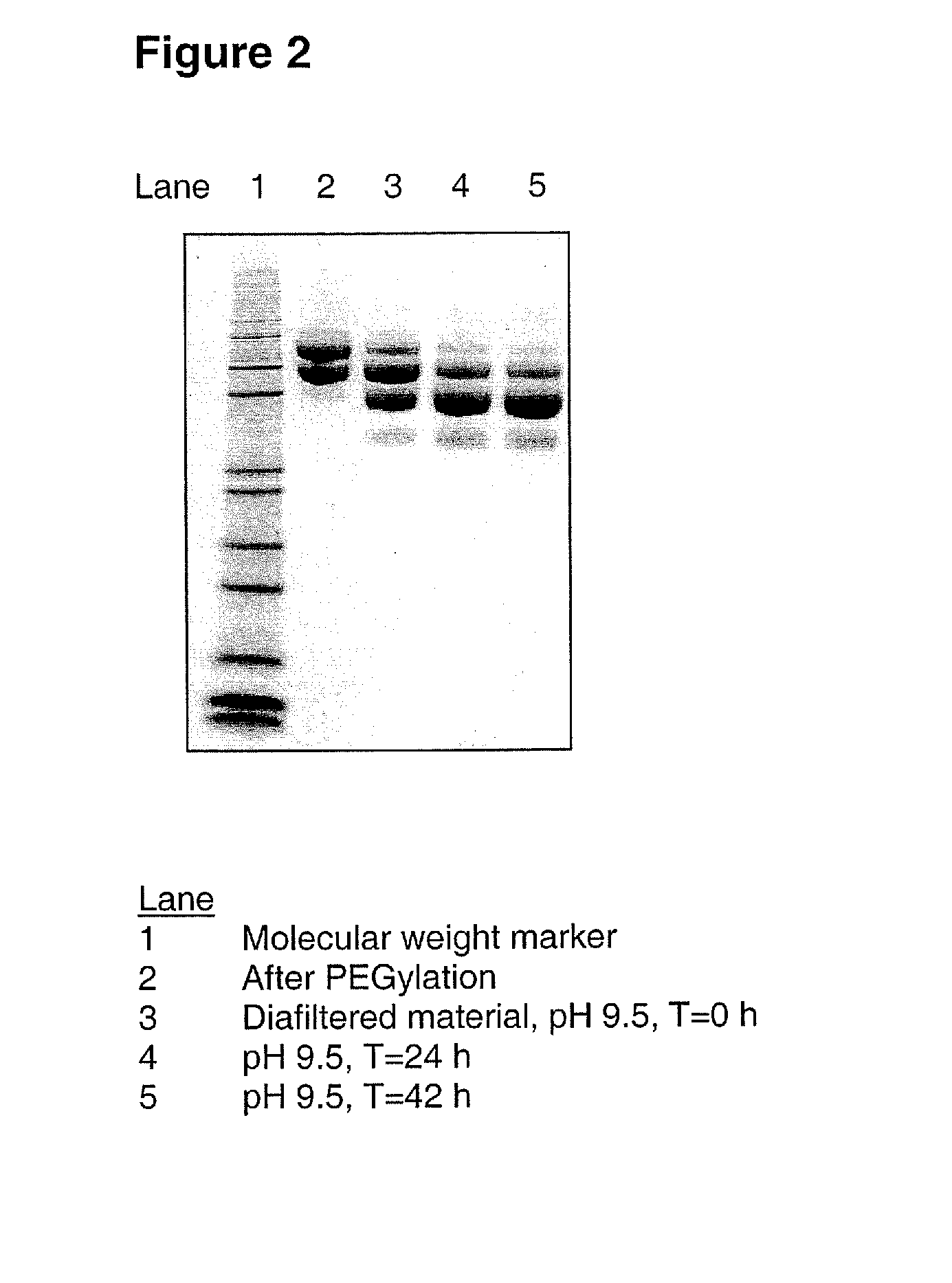
[0101] A G-CSF variant having the substitutions K16R, K34R, K40R, T105K and S159K compared to wild-type human G-CSF (SEQ ID NO:1) was produced from CHO-K1 cells and subsequently PEGylated using mPEG-SPA 5000 substantially as described in Example 1 above, resulting in 29 mL of 3.5 mg / mL PEGylated G-CSF variant. This was diafiltrated into 150 mM sodium borate, pH 9.5, using a Vivaspin filter device equipped with a 10 kDa MWCO filter. The final sample concentration was 4.7 mg / mL. The sample was then incubated at 21±3° C. for 42 hours.
[0102] After incubation, the sample was prepared for cation exchange chromatography by dilution with 30 mM citric acid, 10 mM NaOH, pH 2.9. The sample was then applied onto an XK 16 / 20 column (Amersham Biosciences) packed with 28 mL of SP-Sepharose HP resin. The column was equilibrated with an equilibration buffer of 20 mM citric acid, 1...
example 3
Comparative Example
[0110] For comparative purposes, FIG. 4 shows a cation exchange chromatogram of a purified, PEGylated G-CSF variant that was not subject to de-PEGylation according to the present invention. The G-CSF variant in this case had the same five substitutions compared to native human G-CSF as indicated above in Examples 1 and 2, i.e. K16R, K34R, K40R, T105K and S159K. It was prepared and PEGylated with mPEG-SPA 5000 substantially as described in Example 1, with the exception of the fact that it was not subjected to incubation at pH 9.5 for removal of labile PEG moieties. As a result, the PEGylated variant comprised a mixture of a number of different PEG isomers having 2-6 attached PEG moieties. This mixture of positional isomers was purified using cation exchange chromatography substantially as described in Example 1. A fraction containing primarily 4-5 attached PEG moieties was isolated and, after ultrafiltration and diafiltration substantially as described in Example ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com