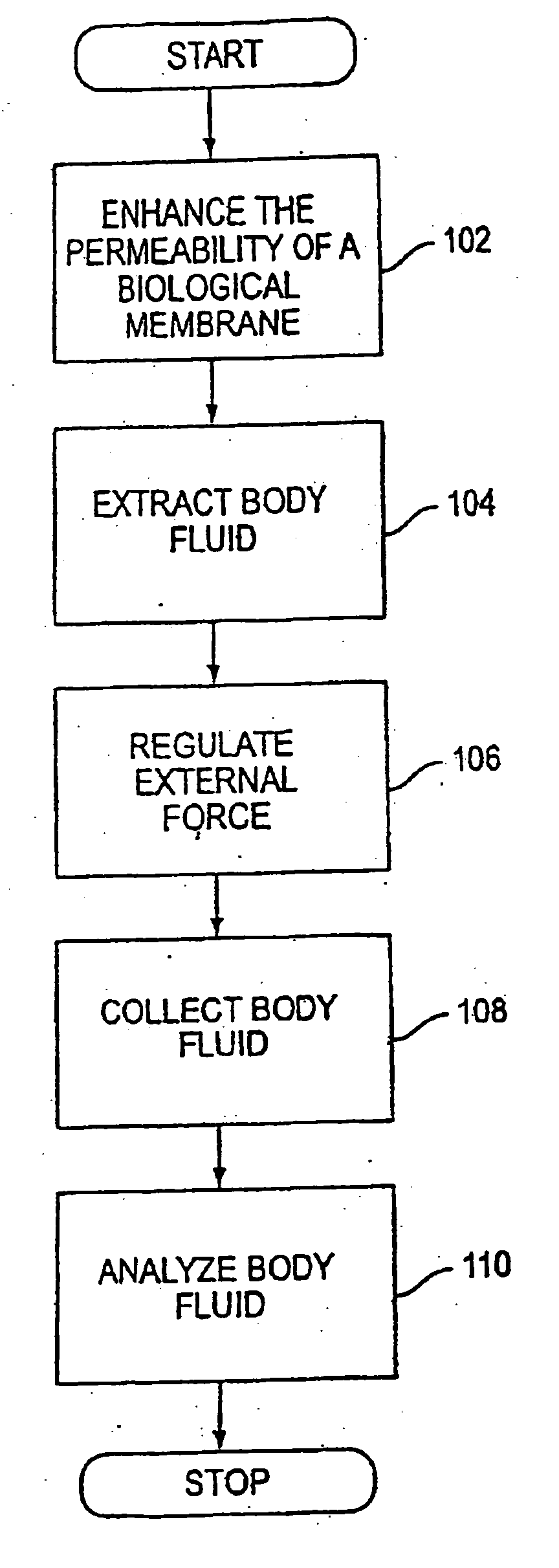
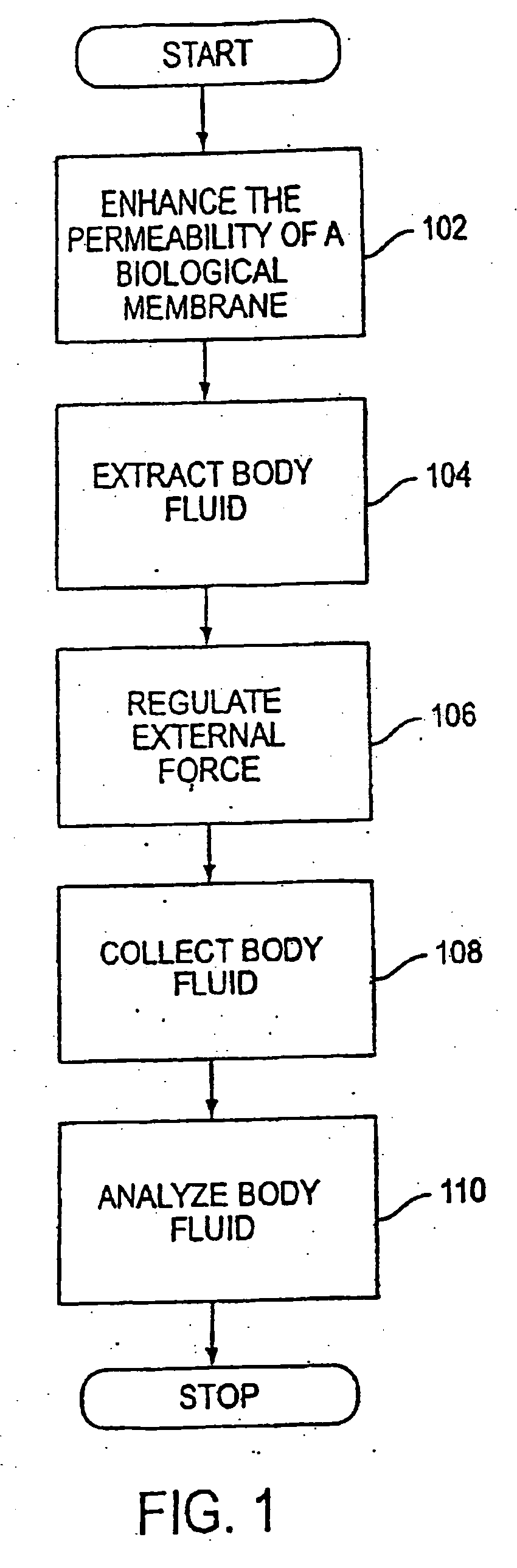
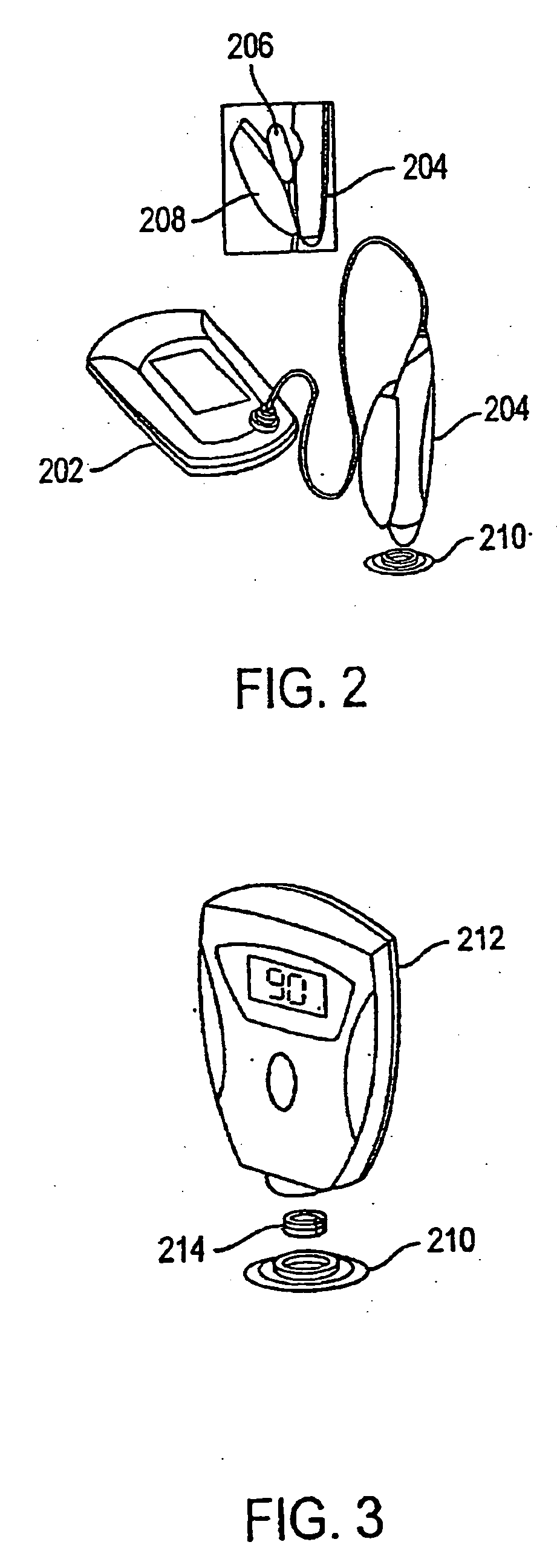
System and method for analyte sampling and analysis
a body fluid and system technology, applied in the field of non-invasive body fluid sampling and analysis, can solve the problems of limited flow and volume of body fluid that can be transported across the stratum comeum, pain and inconvenience of using blood for frequent monitoring, and certain limitations of force applied to human skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0114] The following example does not limit the present invention in any way, and is intended to illustrate an embodiment of the present invention.
[0115] The following is a description of experiments which implemented painless extraction, collection, and analysis of body fluid to determine body fluid glucose concentration in a human using a hyperosmotic extraction fluid and comparing this condition with iso-osmotic extraction fluid, in accordance with one embodiment of the present invention. Although body fluid glucose concentration serves as an example to demonstrate feasibility, other analytes are within the contemplation of the present invention. In addition, multiple analytes may be measured and / or analyzed simultaneously, in parallel, or in series, and results from these multiple measurements may be used in combination with algorithms, for example, to increase the accuracy or precision or both of measurements. As may be recognized by one of ordinary skill in the art, these ste...
example 2
[0160] Vinyl acetate based hydrogels for use with glucose monitoring can be prepared as follows. A 1:1 mixture of n-vinylpyrolidone and vinyl acetate can be polymerized by ultraviolet radiation using 0-0.5% Irgacure as the photoinitiator. A non-woven plastic scrim (such as Delstar product# RB0707-50P) is used to provide mechanic support. The hydrogel's equilibrium water content is 20-95% with its aqueous composition containing 0-1 M sodium or potassium phosphate, 0-1 M sodium chloride, 0-1 M potassium chloride, 0-2 M lactic acid, surfactant such as 0-1 M Triton X-100, Tween 80 or sodium lauryl sulfate, and any other biocompatible components. Glucose oxidase can be loaded by soaking the solid hydrogel layer in concentrated glucose oxidase solution for a period of time.
[0161] A particular example of a vinyl acetate based hydrogel was made with the following constituents: 15% n-vinylpyrolidone, 15% vinyl acetate, 0.05% Irgacure, 0.05 M potassium phosphate, 0.30 M sodium chloride, 0.02...
example 3
[0163] Agarose based hydrogels for use with glucose monitoring were prepared as follows. 0.0116 g of sodium chloride, 0.015 g of potassium chloride, 0.0348 g of dibasic potassium phosphate and 0.002 g of Triton X-100 were dissolved in 10 mL of water. The pH of the solution was adjusted to 7.0 using 0.5 M hydrochloric acid with the aid of a pH meter. The solution was diluted with water to 20 mL. This was Solution A. 0.2 g of agarose powder was mixed and dispersed in Solution A. Agarose was heated and dissolved until boiling in a water bath. This was Solution B. Solution B was allowed to cool down to 35° C. 0.01 g of glucose oxidase powder was completely mixed and dissolved in Solution B. This was Solution C. Solution C was cast and filled onto a warm, flat mold surface. The mold was transferred to room temperature or lower to form gels.
[0164]FIG. 12 shows sensor signal response as a function of glucose concentration for two types of agarose hydrogels relative to a polyethylene oxide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Biological properties | aaaaa | aaaaa |
| Permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com