Anhydrous flouride salts and reagents and methods for their production
an anhydrous flouride salt and reagent technology, applied in the field of new organic fluorides, can solve the problems of -hydrogen atoms, inability to prepare compounds according to current methodologies, and inability to meet the requirements of current methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0035] All reagents were handled under N2. Hexafluorobenzene (C6F6) (99%, SynQuest) was passed through a column of activated (130° C. for 5 h) silica gel and distilled from CaH2. Acetonitrile (HPLC grade, Aldrich) was distilled from P2O5 and redistilled under reduced pressure from CaH2. THF (anhydrous, Aldrich) was distilled from LiAlH4. Purified solvents were stored under N2 in Schlenk-style flasks under N2. Tetra-n-butylammonium cyanide (TBACN) (97%) was obtained from Fluka Chemical Co. TBACN was dried under vacuum at 40° C. overnight prior to use. For initial work, TBACN was recrystallized from THF / Hexane by layering, subsequent studies showed that this purification step was unnecessary. Tetramethylammonium hexafluorophosphate (TMAPF6) was obtained from Fluka and dried under vacuum. All other reagents were of analytical grade, from Aldrich. All chemical handling was performed under N2 in a glove box.
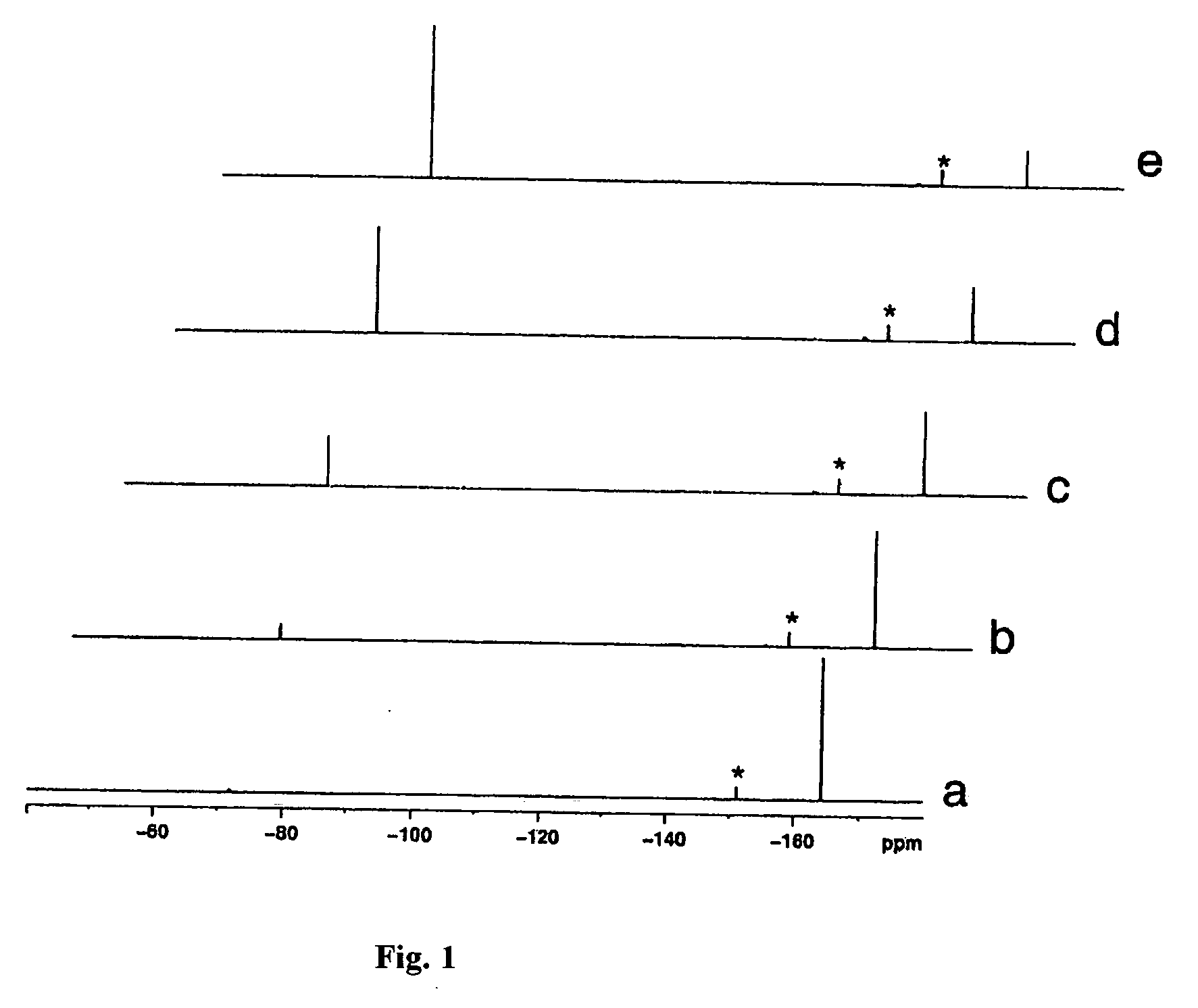
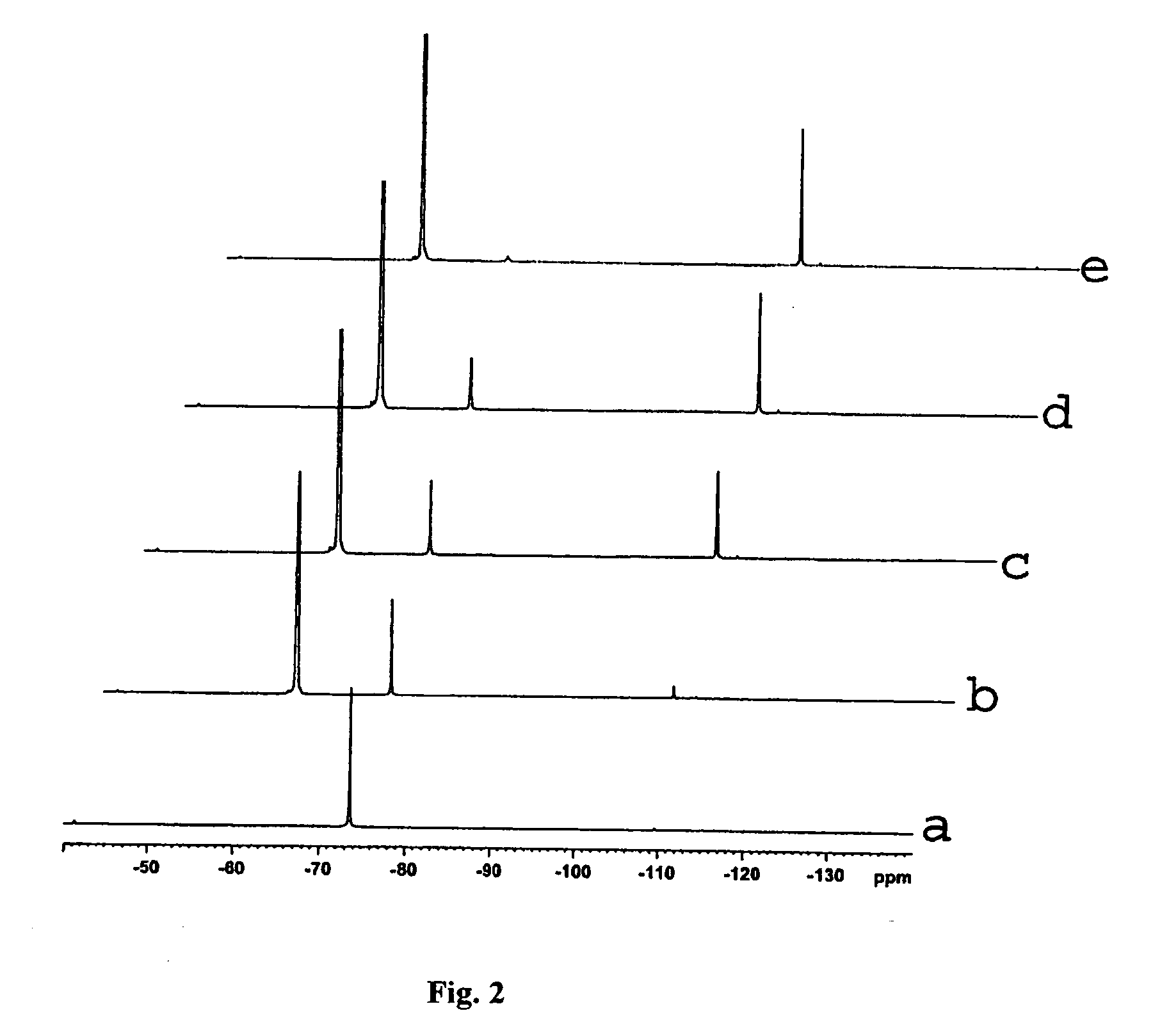
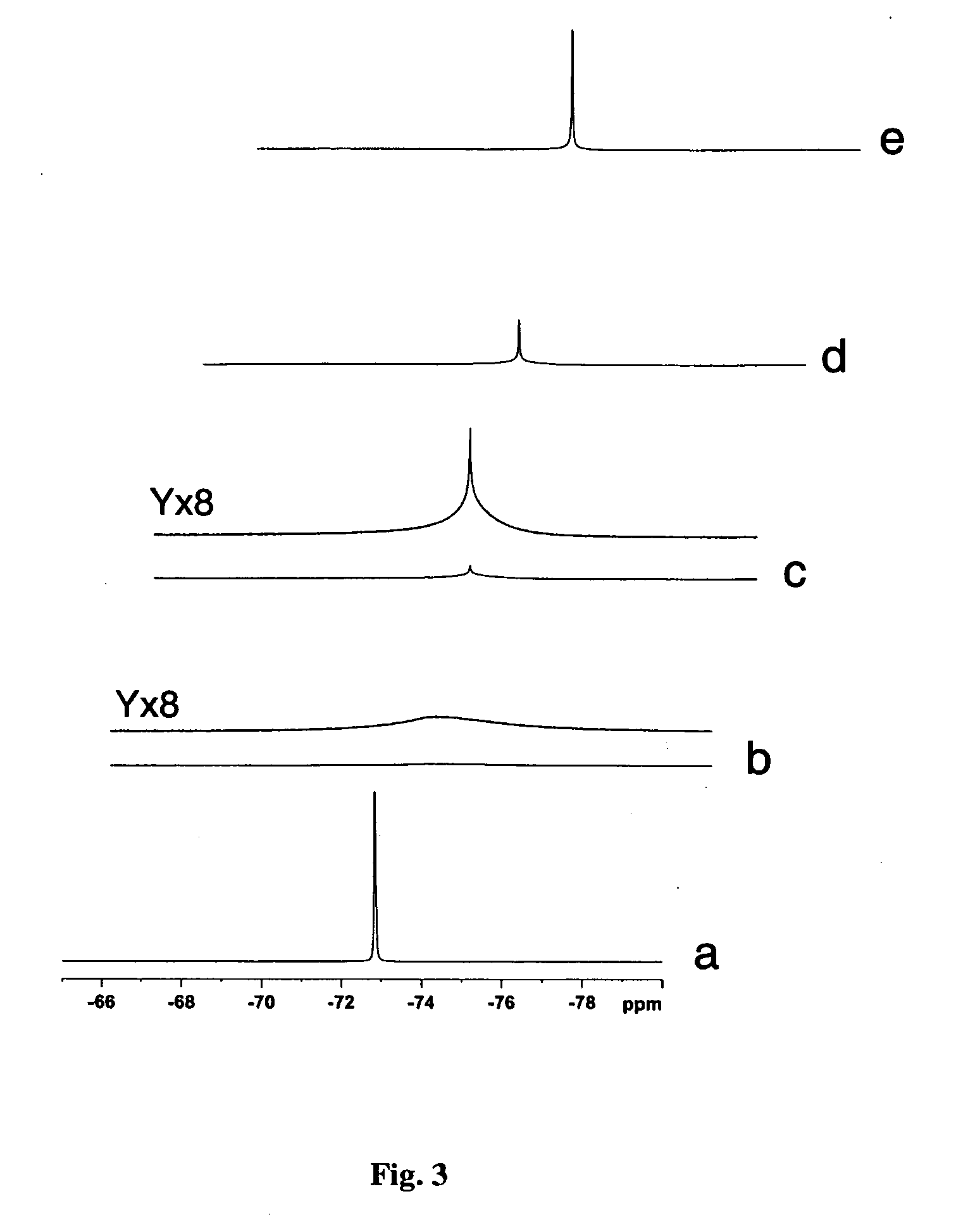
[0036]1H, 13C and 19F NMR spectra were determined in the Instrumentation Center ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com