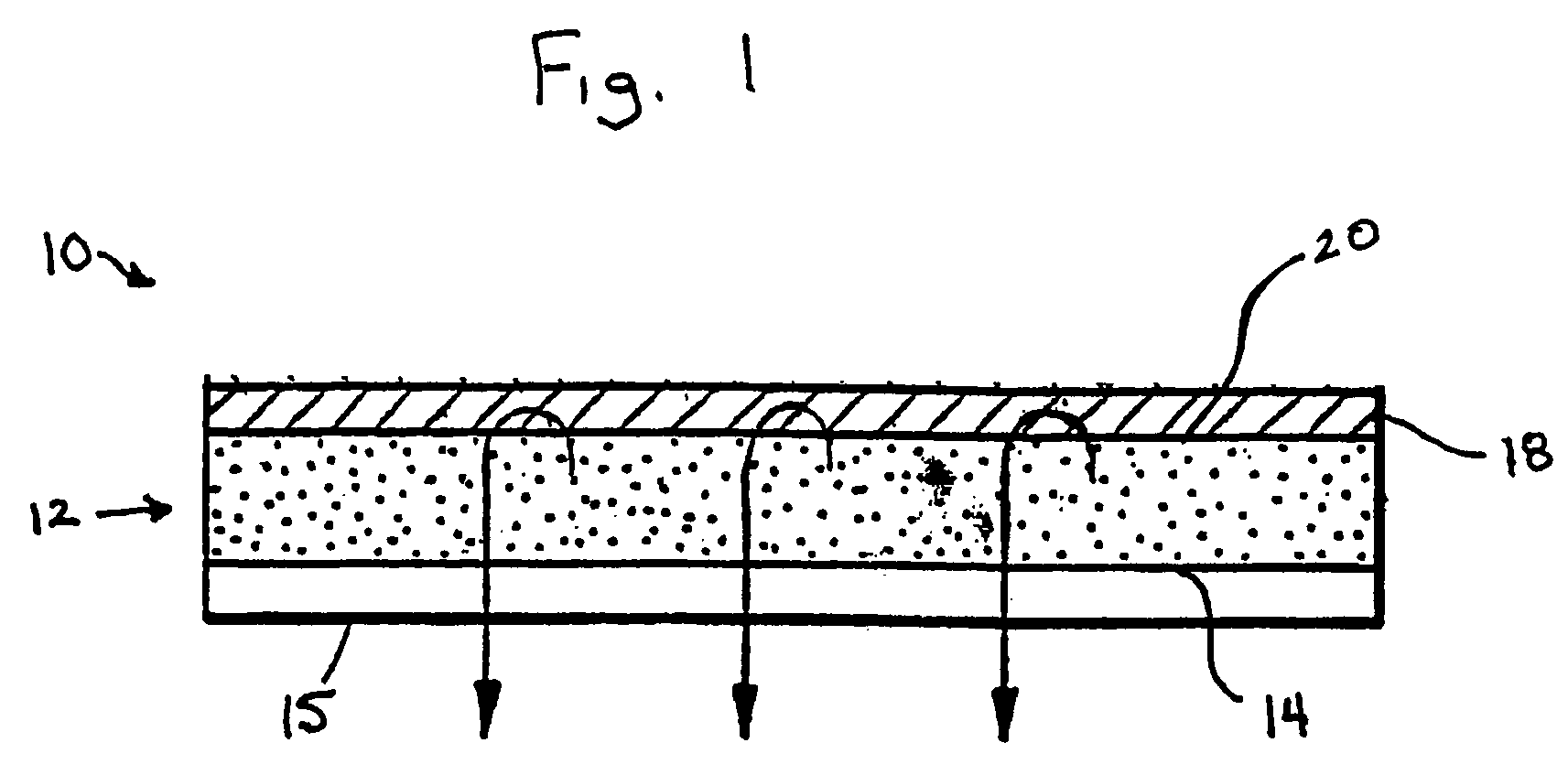
Transdermal drug delivery device including an occlusive backing
a transdermal drug and occlusive backing technology, applied in the direction of anti-inflammatory agents, cardiovascular disorders, drug compositions, etc., can solve the problems of limiting the use of transdermal administration, non-uniform drug release profiles, and impractical to increase the duration of the device's application while retaining therapeutic effectiveness, etc., to achieve convenient and convenient use, convenient use, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
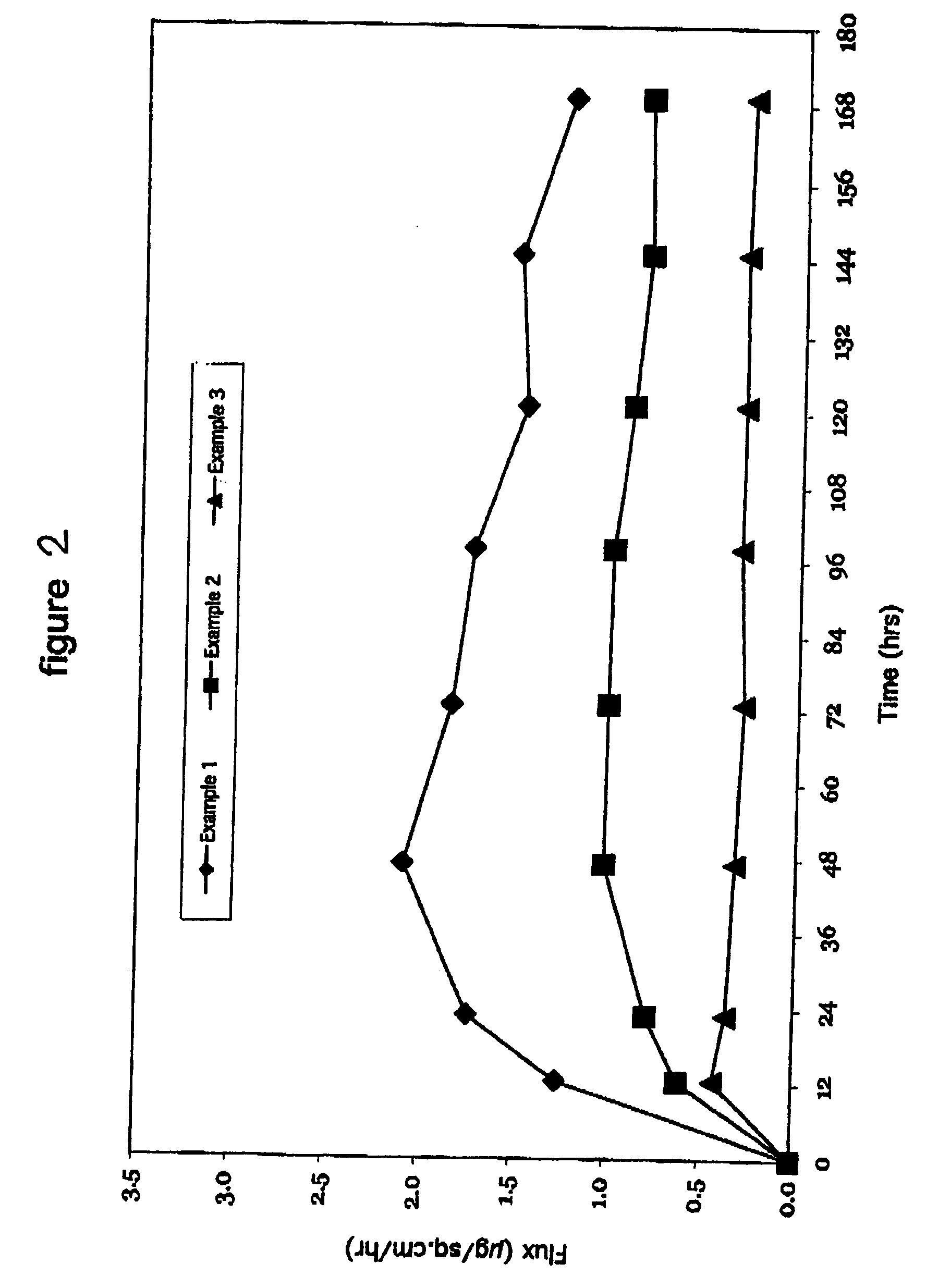
[0418] In Example 1, a backing layer comprising polyester and ethylene vinyl acetate was used. The backing layer is commercially available from 3M as ScotchPak 9732. The backing layer had a moisture vapor transmission rate of 15.5 g / m2 / 24 hours. The maxtix blend which included 7% by weight clonidine, 83% by weight of a non-functional, acrylic-based pressure sensitive adhesive (DuroTak 73-9301) and 10% by weight of a carboxy functional acrylic-based pressure sensitive adhesive (DuroTak 87-2852) was formed over the backing layer. The matrix was identical to that used in Examples 1 and 3. As can be seen in Table 3, the flux of the transdermal delivery device was 1.69 μg / cm2 / hr.
example 2
[0419] In Example 2, a backing layer comprising polyurethane and ethylene vinyl alcohol commercially available from J.P. Stevens Co. of East Hampton, Mass. was used. The backing layer had a moisture vapor transmission rate of 100 g / m2 / 24 hours. The maxtix blend which included 7% by weight clonidine, 83% by weight of a non-functional, acrylic-based pressure sensitive adhesive (DuroTak 73-9301) and 10% by weight of a carboxy functional acrylic-based pressure sensitive adhesive (DuroTak 87-2852) was formed over the backing layer. The matrix was identical to that used in Examples 1 and 3. As can be seen in Table 3, the flux of the transdermal delivery device was 0.93 μg / cm2 / hr.
example 3
[0420] In Example 3, a polyurethane backing layer commercially available from J.P. Stevens Co. of East Hampton, Mass. was used. The backing layer had a moisture vapor transmission rate of 1500 g / m2 / 24 hours. The maxtix blend included 7% by weight clonidine, 83% by weight of a non-functional, acrylic-based pressure sensitive adhesive (DuroTak 73-9301) and 10% by weight of a carboxy functional acrylic-based pressure sensitive adhesive (DuroTak 87-2852) was formed over the backing layer and was identical to that used in Examples 1 and 2. As can be seen in Table 3, the flux of the transdermal delivery device was 0.32 μg / cm2 / hr.
TABLE 3Backing MaterialMVTR (g / m2 / 24 hrs)Flux (μg / cm2 / hr)Example 1PET / EVA15.51.69Example 2PU / EVOH1000.93Example 3PU15000.32
[0421] The results from Examples 1 to 3 are set forth graphically in FIG. 2. As illustrated in the forgoing Examples and in FIG. 2, backing layers having a low water vapor transmission rates increase the flux of clonidine. As such, varying t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com