Noble metal nanotube and method for preparation thereof
a nanotube and noble metal technology, applied in the field of new metals having a nanotube structure, can solve the problems of difficult to achieve, and no previous cases of applying such complex effects to the template synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0041] Nonaethylene glycol monododecyl ether (C12 EO9) was dropped into aqueous solution of chloroplatinic acid (H2PtCl6) taken in a test tube and was heated to 60° C. Further, polyoxyethylene (20) sorbitan monostearate (tween60, trade name; available from Wako Pure Chemical Industries, Ltd.) was added. After the test tube was shaken in water bath of 60° C. for three minutes, the test tube was left in air constant-temperature bath of 25° C. for two minutes. This procedure was repeated three times.
[0042] The test tube was left at 25° C. for twenty minutes, thereby preparing reaction mixture of feeding molar ratio H2PtCl6:C12EO9:tween60:H2O=1:1:1:60. Hydrazine of which molar ratio was 16 times as much as the chloroplatinic acid was dropped into the reaction mixture at the same temperature and was reacted for 24 hours. Fine solid phase deposition was centrifuged, after that, washed with water, then washed with ethanol, and dried, thereby obtaining black powder.
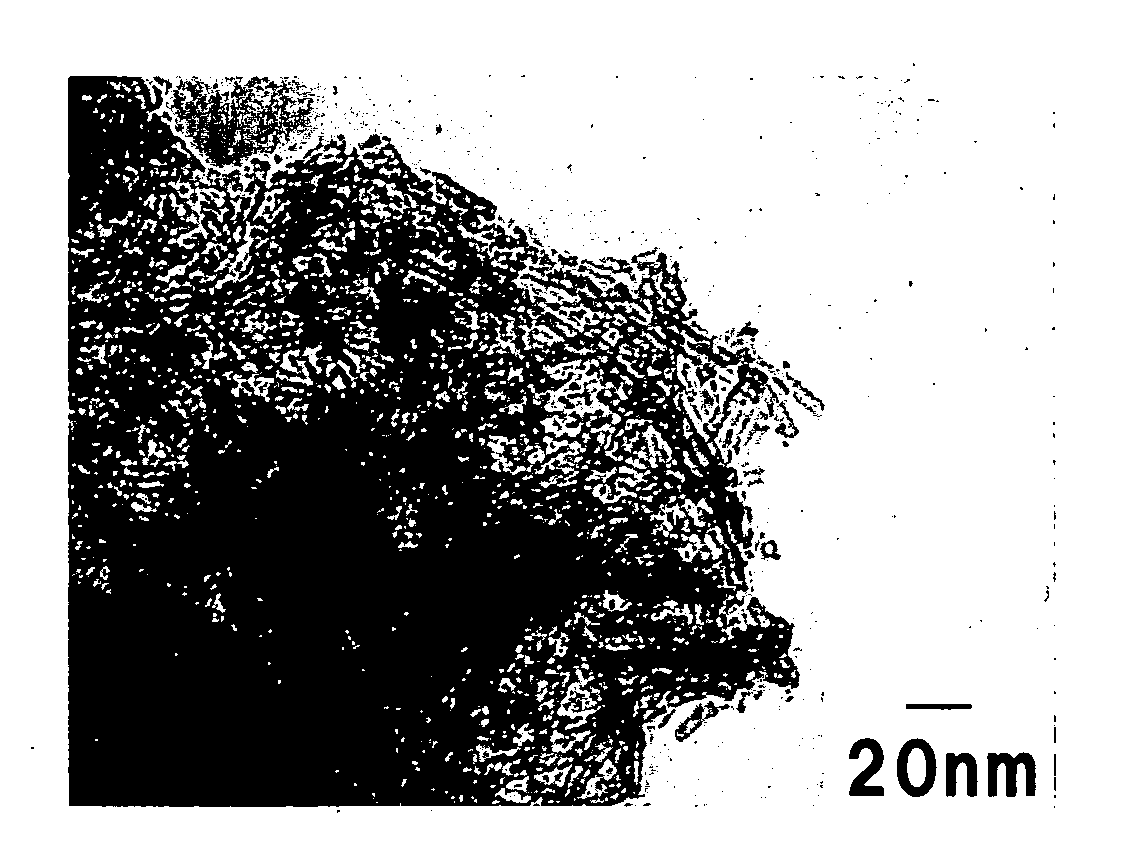
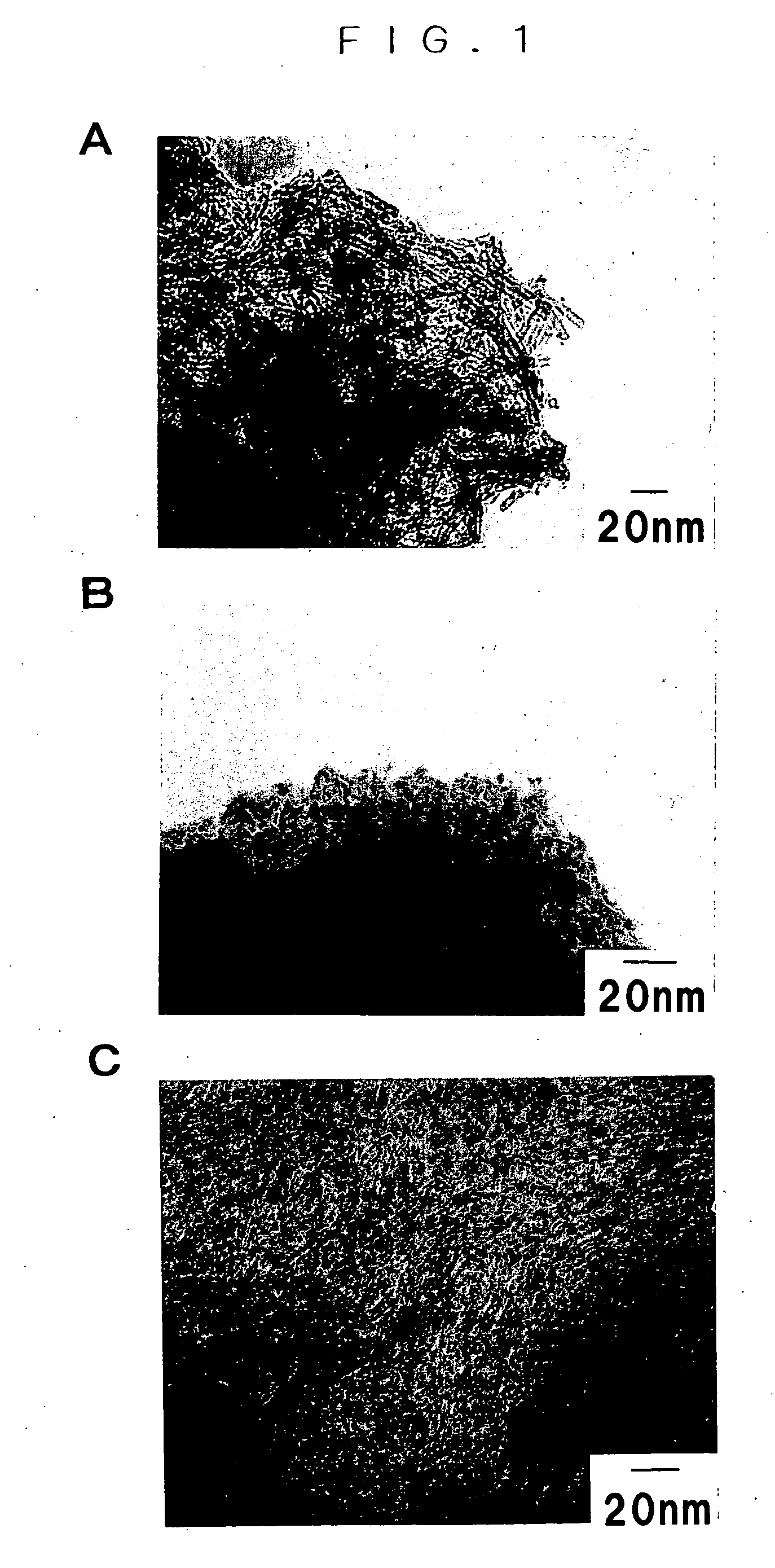
[0043] The powder was o...
example 2
[0044] Nonaethylene glycol monododecyl ether (C12 EO9) was dropped into aqueous solution of palladium chloride (PdCl2) taken in a test tube and was heated to 60° C. Further, polyoxyethylene (20) sorbitan monostearate (tween60, trade name; available from Wako Pure Chemical Industries, Ltd.) was added. After the test tube was shaken in water bath of 60° C. for 15 minutes, the test tube was cooled to 25° C. and left at this temperature for twenty minutes, thereby preparing reaction mixture of feeding molar ratio PdCl2:C12EO9:tween60:H2O=1:1:1:60. Hydrazine of which molar ratio was 16 times as much as the palladium chloride was dropped into the reaction mixture at the same temperature and was reacted for 24 hours.
[0045] Fine solid phase deposition was centrifuged, after that, washed with water, then washed with ethanol, and dried, thereby obtaining black powder.
[0046] The powder was observed by the transmission electron microscope and it was confirmed that the major product of the pow...
example 3
[0047] Sodium dodecylsulfate (SDS) was added to 0.056M nitric acid solution of silver nitrate (AgNO3) taken in a test tube and was heated to 60° C. so as to obtain uniform solution. Further, polyoxyethylene (20) sorbitan monostearate (tween60, trade name; available from Wako Pure Chemical Industries, Ltd.) was added to the solution. After the test tube was shaken for ten minutes, the test tube was cooled to 25° C., thereby preparing reaction mixture of feeding molar ratio AgNO3:SDS:tween60:H2O (0.056M HNO3)=1:1:1:60. Hydrazine of which molar ratio was 16 times as much as the silver nitrate was dropped into the reaction mixture at the same temperature and was reacted for 24 hours. Fine solid phase deposition was centrifuged, after that, washed with water, then washed with ethanol, and dried, thereby obtaining gray powder.
[0048] The powder was observed by the transmission electron microscope and it was confirmed that the major product of the powder was tubular particle of about 7 nm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nanoscale particle size | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com