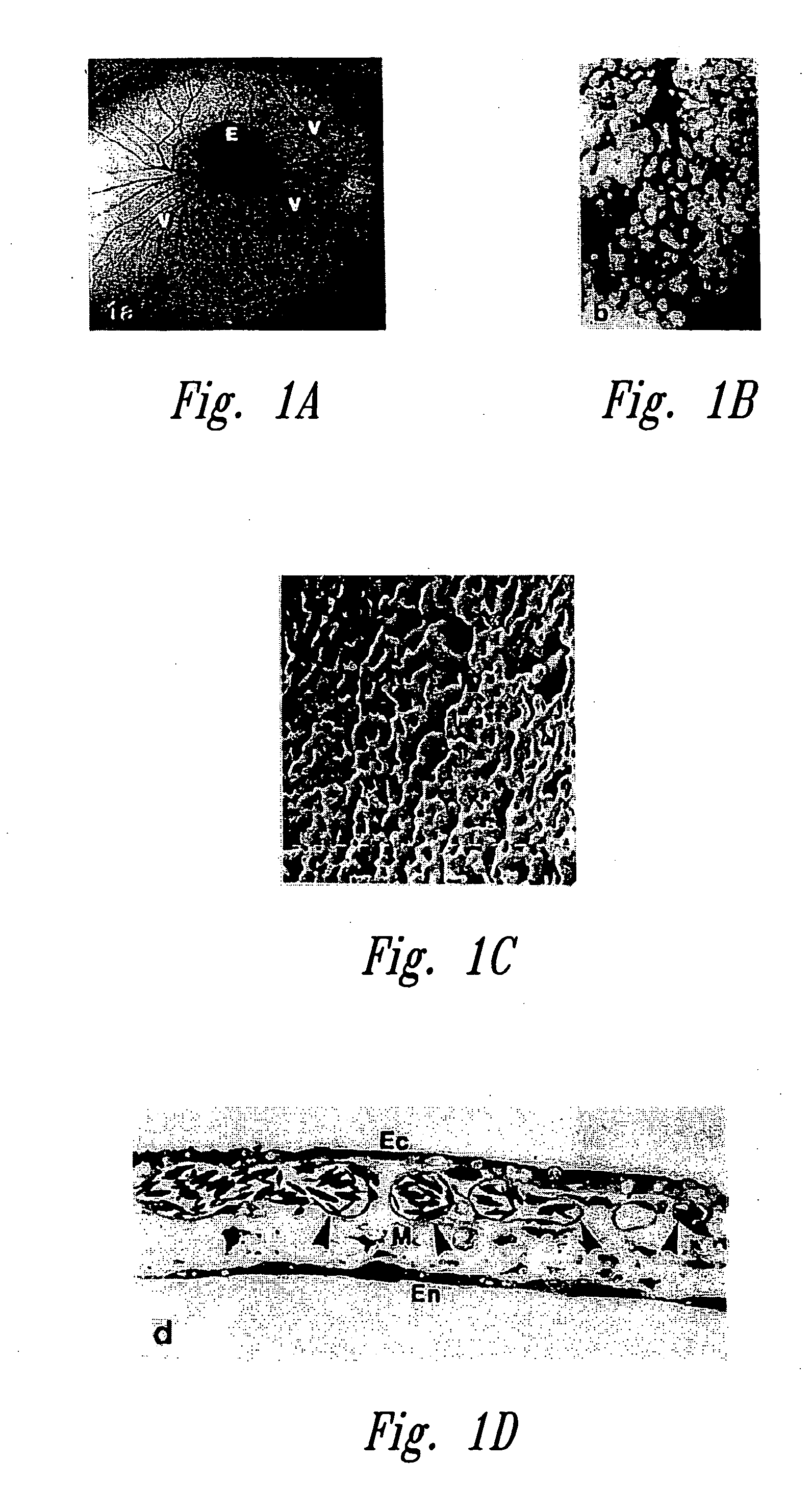
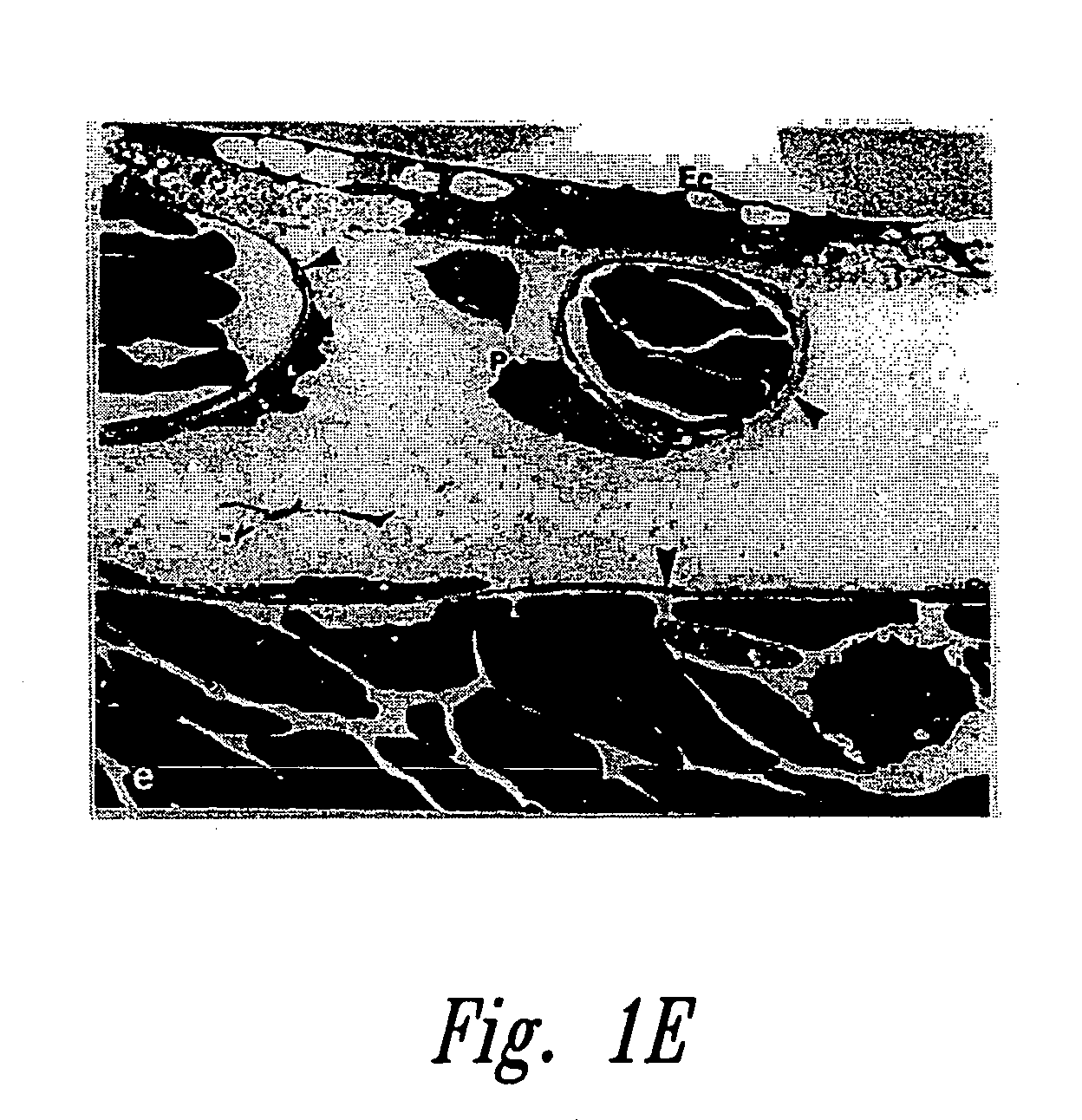
Anti-angiogenic compositions and methods of use
a technology of compositions and compositions, applied in the field of compositions and methods for treating cancer and other angiogenicdependent diseases, can solve the problems of radiotherapy and chemotherapy agents being toxic to normal tissues, having life-threatening side effects, and often having extremely high failure/remission rates, and achieve the effect of occludemen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Anti-Invasive Factor
[0220] The shoulder girdle and skull from a dogfish is excised, then scraped with a scalpel in order to remove all muscle and associated connective tissue from the cartilage. The cartilage is then homogenized with a tissue grinder, and extracted by continuous stirring at room temperature for 2 to 5 days in a solution containing 2.0 M guanidium hydrochloride and 0.02 M MES at pH 6.0.
[0221] After 2 to 5 days, the cartilage extract is passed through gauze netting in order to remove the larger constituents. The filtrate is then passed through an Amicon ultrafiltration unit which utilizes spiral-wound cartridges, with a molecular weight cutoff of 100,000. The filtrate (containing proteins with a molecular weight of less than 100,000 daltons) is then dialyzed against 0.02 M MES buffer (pH 6) with an Amicon ultrafiltration unit which retains proteins with a molecular weight of greater than 3,000 daltons. Utilizing this method, low molecular weight prote...
example 2
Analysis of Various Agents for Anti-Angiogenic Activity
[0222] A. Chick Chorioallantoic Membrane (“Cam”) Assays
[0223] Fertilized, domestic chick embryos were incubated for 3 days prior to shell-less culturing. In this procedure, the egg contents were emptied by removing the shell located around the air space. The interior shell membrane was then severed and the opposite end of the shell was perforated to allow the contents of the egg to gently slide out from the blunted end. The egg contents were emptied into round-bottom sterilized glass bowls and covered with petri dish covers. These were then placed into an incubator at 90% relative humidity and 3% CO2 and incubated for 3 days.
[0224] Paclitaxel (Sigma, St. Louis, Mich.) was mixed at concentrations of 1, 5, 10, 30 μg per 10 ml aliquot of 0.5% aqueous methylcellulose. Since paclitaxel is insoluble in water, glass beads were used to produce fine particles. Ten microliter aliquots of this solution were dried on parafilm for 1 hour ...
example 3
Encapsulation of Suramin
[0237] One milliliter of 5% ELVAX (poly(ethylene-vinyl acetate) cross-linked with 5% vinyl acetate) in dichloromethane (“DCM”) is mixed with a fixed weight of sub-micron ground sodium suramin. This mixture is injected into 5 ml of 5% Polyvinyl Alcohol (“PVA”) in water in a 30 ml flat bottomed test tube. Tubes containing different weights of the drug are then suspended in a multi-sample water bath at 40° for 90 minutes with automated stirring. The mixtures are removed, and microsphere samples taken for size analysis. Tubes are centrifuged at 1000 g for 5 min. The PVA supernatant is removed and saved for analysis (nonencapsulated drug). The microspheres are then washed (vortexed) in 5 ml of water and recentrifuged. The 5 ml wash is saved for analysis (surface bound drug). Microspheres are then wetted in 50 ul of methanol, and vortexed in 1 ml of DCM to dissolve the ELVAX. The microspheres are then warmed to 40° C., and 5 ml of 50° C. water is slowly added with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| survival time | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com