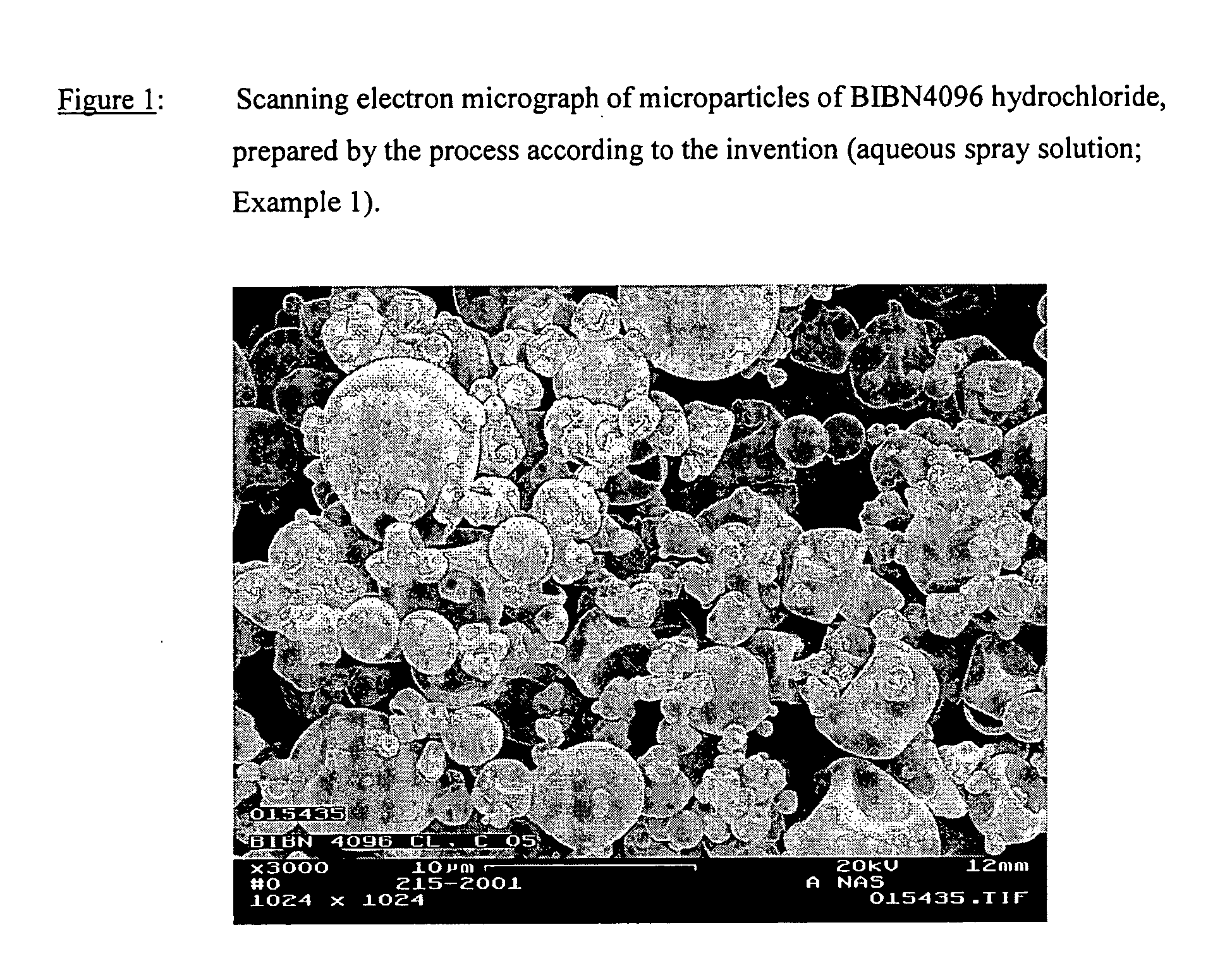
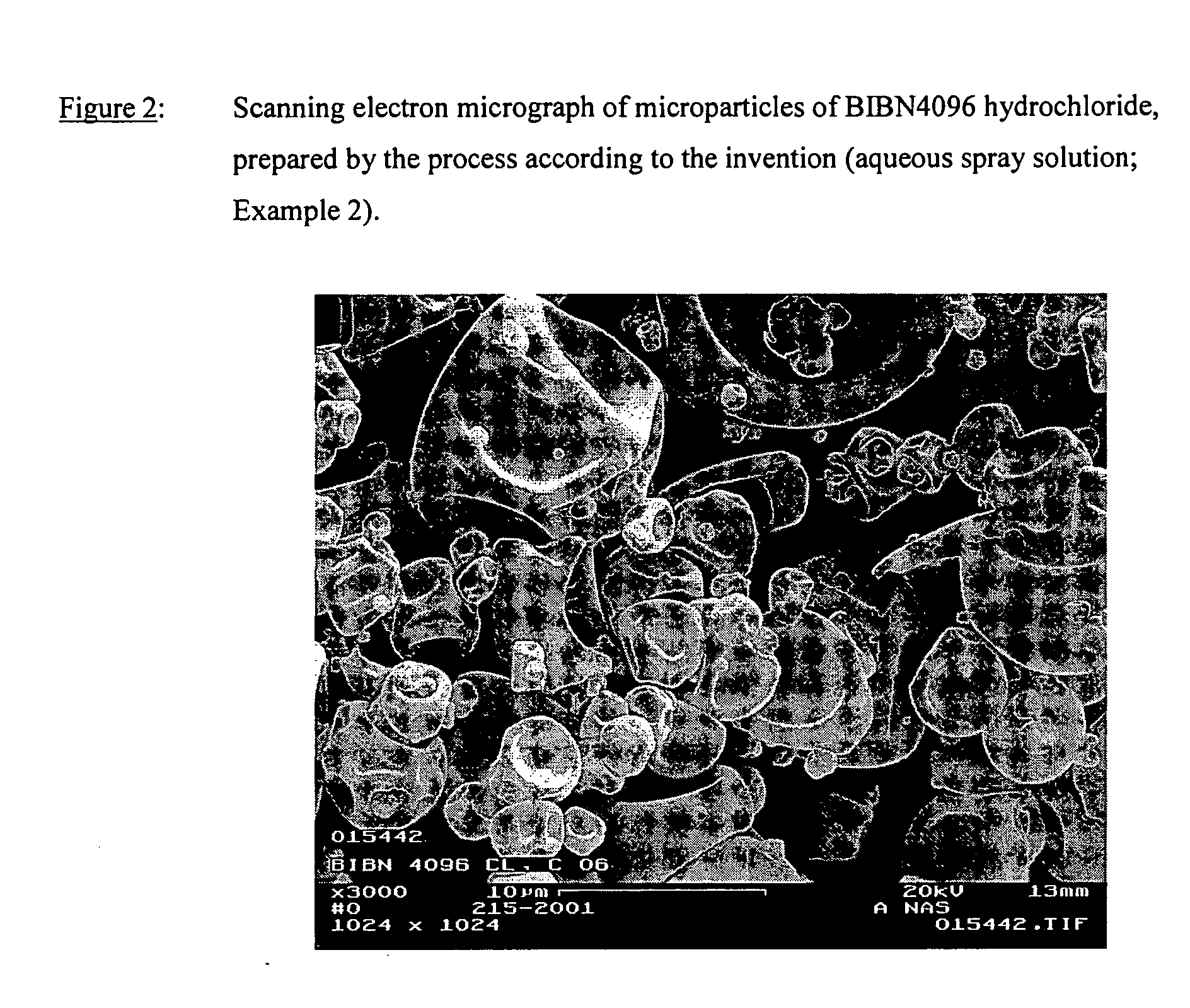
Salts of the CGRP antagonist BIBN4096 and inhalable powdered medicaments containing them
a technology of bibn4096 and bibn4096, which is applied in the field of salts, can solve the problems of inability to administer orally using conventional preparations, increased stress, and compatibility problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
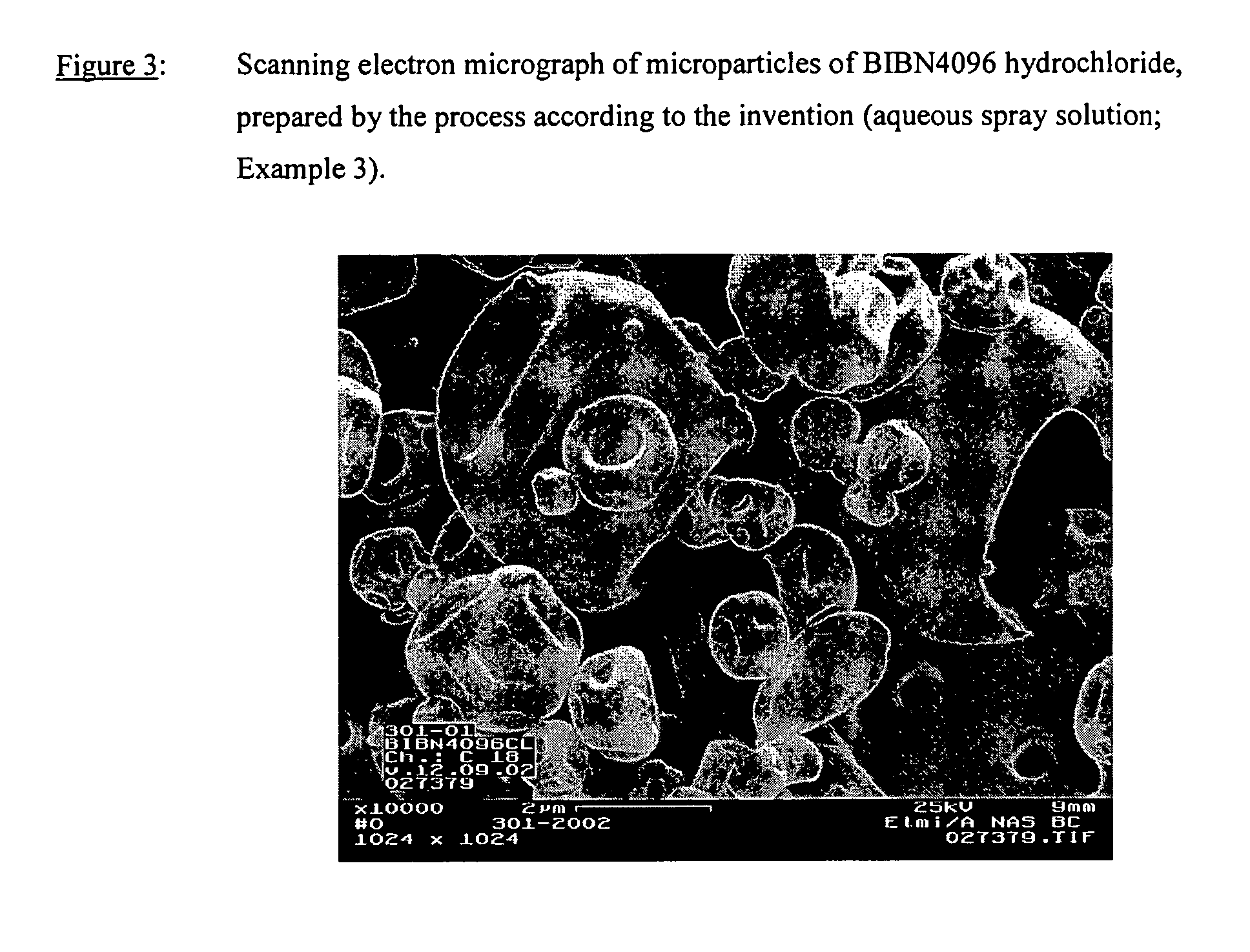
Image
Examples
example 5
Spray Parameter Suitable for an Aqueous BIBN4096 Solution (Modified BÜCHI Spray Tower)
[0084]
Concentration solution2 g / 100 gsolvent:0.012 mol / L sulphuric acidDroplet size Q(5.8)39%(Reference solution: H2O at ambienttemperature)X507.0 μmvolumetric flow “spray rate”0.54 L / hspray pressure3.3-3.5 bar overpressure (N2)(nozzle type)(BÜCHI spray nozzle 0.7 mm,Art. No. 04364)Mass flow Spray gas nozzle type2.1 kg / h(BÜCHI spray nozzle 0.7 mm,Art. No. 04364)Inlet temperature150° C.Outlet temperature101° C.volumetric flow “drying gas”35 standard m3 / hcross section of drying tower105 mm
example 6
Spray Parameter Suitable for an Qqueous BIBN4096 Solution (Modified BÜCHI Spray Tower)
[0085]
Concentration solution5 g / 100 gsolvent:0.02 mol / L citric acidDroplet size Q(5.8)55%(Reference solution: H2O at ambienttemperature)X505.5 μmvolumetric flow “spray rate”0.54 L / hspray pressure4.4-4.6 bar overpressure (N2)(nozzle type)(BÜCHI spray nozzle 0.7 mm,Art. No. 04364)Mass flow Spray gas nozzle type2.8 kg / h(BÜCHI spray nozzle 0.7 mm,Art. No. 04364)Inlet temperature150° C.Outlet temperature 99° C.volumetric flow “drying gas”35 standard m3 / hcross section of drying tower105 mm
example 7
Spray Parameter Suitable for an Aqueous BIBN4096 Solution (Modified BÜCHI Spray Tower)
[0086]
Concentration solution10 g / 100 gsolvent:0.06 mol / L tartaric acidDroplet size Q(5.8)60%(Reference solution: H2O at ambienttemperature)X505.3 μmvolumetric flow “spray rate”0.54 L / hspray pressure4.7-4.9 bar overpressure (N2)(nozzle type)(BÜCHI spray nozzle 0.7 mm,Art. No. 04364)Mass flow Spray gas nozzle type3 kg / h(BÜCHI spray nozzle 0.7 mm,Art. No. 04364)Inlet temperature150° C.Outlet temperature101° C.volumetric flow “drying gas”35 standard m3 / hcross section of drying tower105 mm
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com