Compositions for in vitro amplification of nucleic acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Real-Time TaqMan PCR in the Presence of Varying Amounts of Anti-Foam
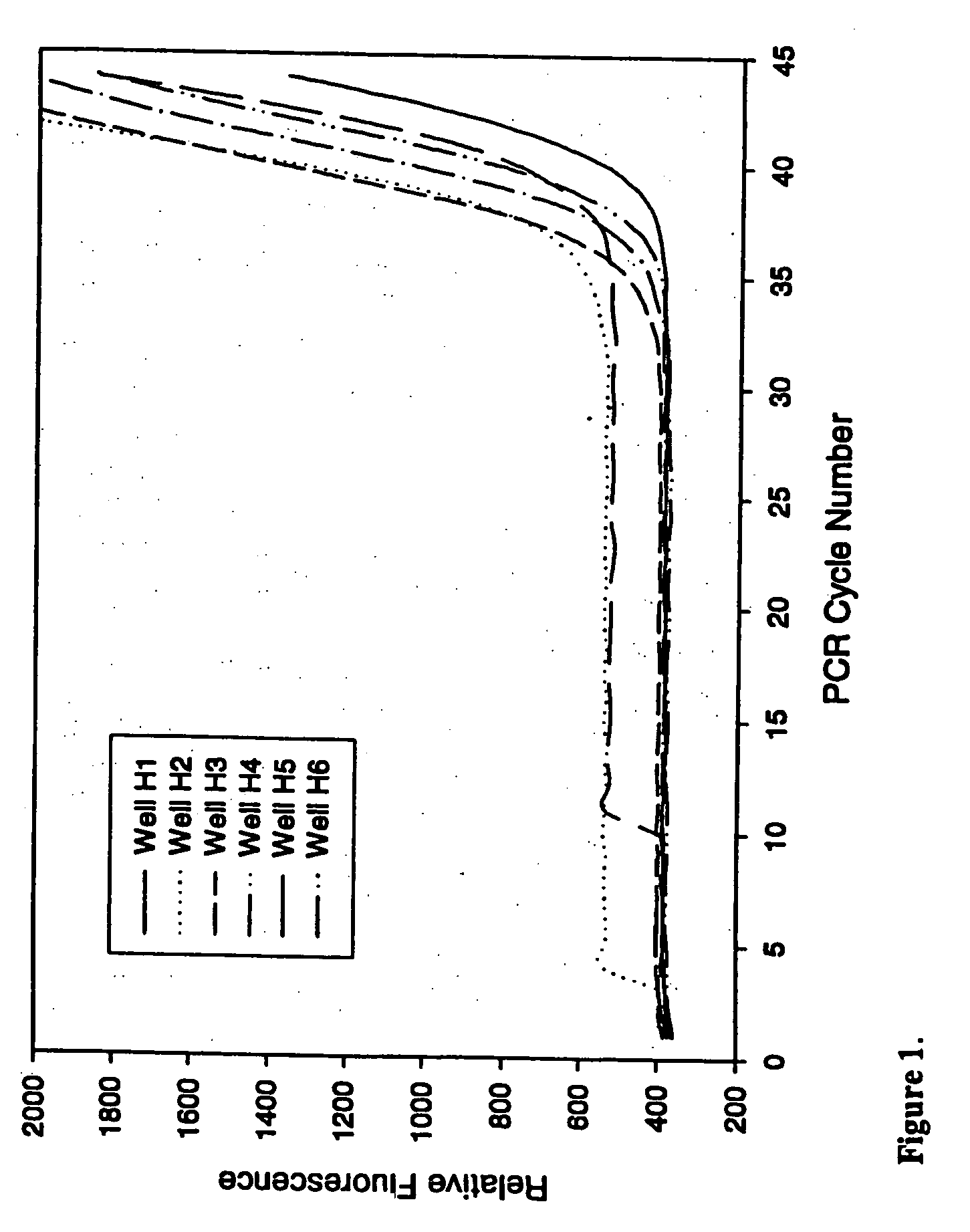
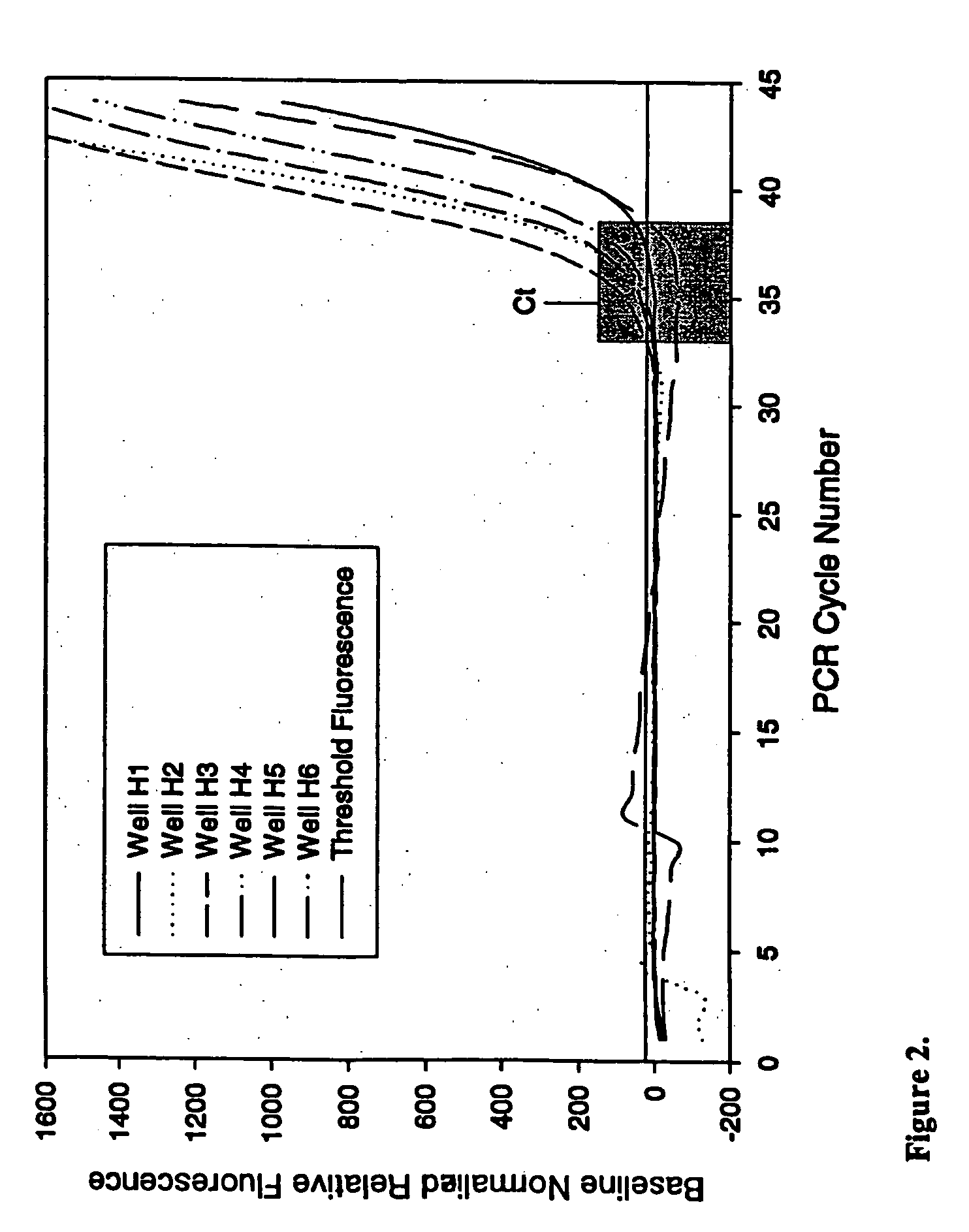
[0077] This example demonstrates the ability of PCR to proceed in the presence of an anti-foam compound.
[0078] Real-time quantitative polymerase chain reactions specific for the human cytoplasmic β-actin sequence were carried out using a commercial hot-start Taq DNA polymerase reaction cocktail as follows. Each 50 μl PCR contained 1×iQ PCR SuperMix (Bio-Rad Laboratories), 200 nM each primer, and 100 nM FAM and TAMRA labeled 5′-nuclease probe as described by Xu et al. 2000, Focus 22:3-5 (forward primer: 5′-CCTGGCACCCAGCACAAT-3′; reverse primer: 5′-GGGCCGGACTCGTCATAC-3′; Taqman probe: 5′-FAM-AGCCGCCGATCCACACGGAGT-TAMRA-3′), and varying amounts of a DNA target. Triplicate PCRs were performed for each amount of input DNA (1×102, 1×104, 1×106, or 1×108 copies of a plasmid containing the gene encoding human cytoplasmic β-actin). PCRs were conducted under identical conditions except for the inclusion of varying amounts (...
example ii
Real-Time TaqMan PCR in the Presence of Different Anti-Foam Compounds
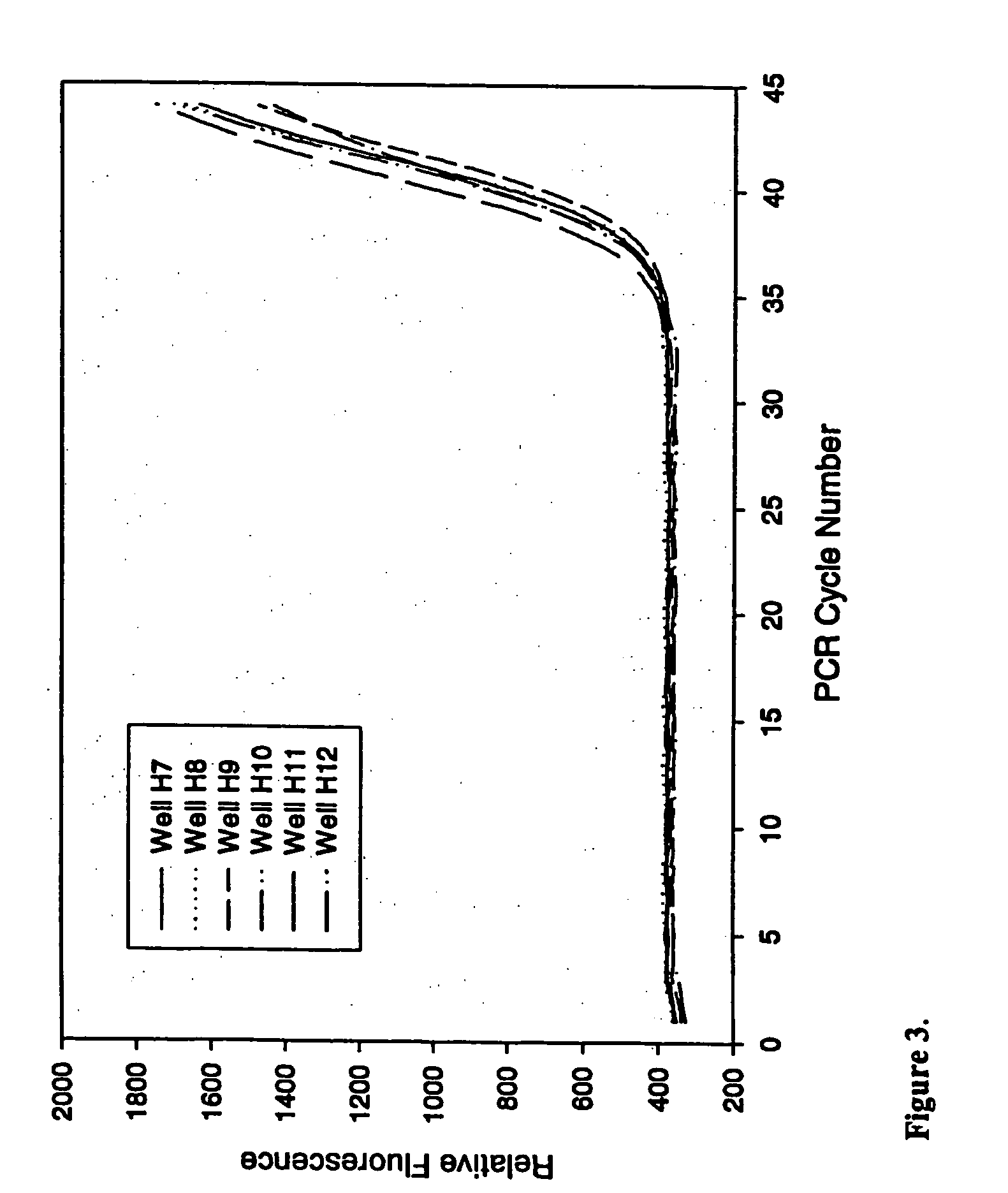
[0081] This example demonstrates the ability of PCR to proceed in the presence of a variety of anti-foam compounds.
[0082] Real-time quantitative polymerase chain reactions specific for the human cytoplasmic β-actin sequence were carried out as described above in example 1. PCRs were conducted under identical conditions except for the inclusion of 0.005% of anti-foam selected from different commercially available preparations from Dow Corning (1520-US, AF, or FG-10) or Sigma (O-30, SE-15, or Antifoam B). Control reactions omitted anti-foam. Results are summarized in table 2.
[0083] Results demonstrated that a variety of anti-foams with different chemical compositions, fatty acid ester (Sigma O-30), or silicone emulsions comprised of polydimethylsiloxane, and emulsion formulations are effective at suppressing foaming by PCR surfactants without adverse effect on DNA amplification. For all anti-foams tested, the pres...
example iii
Real-Time SYBR Green I PCR in the Presence of Antifoam Reagent
[0084] This example extends the efficacy and compatibility of anti-foam in real-time PCR using different homogeneous detection chemistries, specifically, fluorescent dsDNA-specific dyes.
[0085] Real-time quantitative polymerase chain reactions specific for the human cytoplasmic β-actin sequence were carried out using a commercial hot-start Taq DNA polymerase reaction cocktail modified for use with SYBR Green I as follows. Each 50 μl PCR contained 1×iQ PCR SuperMix (Bio-Rad Laboratories), 2% DMSO, 0.25×SYBR Green I (Molecular Probes), 300 nM each primer, as described by Xu et al. 2000, Focus 22:3-5 (forward primer: 5′-CCTGGCACCCAGCACAAT-3′; reverse primer: 5′-GGGCCGGACTCGTCATAC-3′), and varying amounts of a DNA target. Triplicate PCRs were performed for each amount of input DNA (1×102, 1×104, 1×106, or 1×108 copies of a plasmid containing the gene encoding human cytoplasmic β-actin). PCRs were conducted under identical co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com