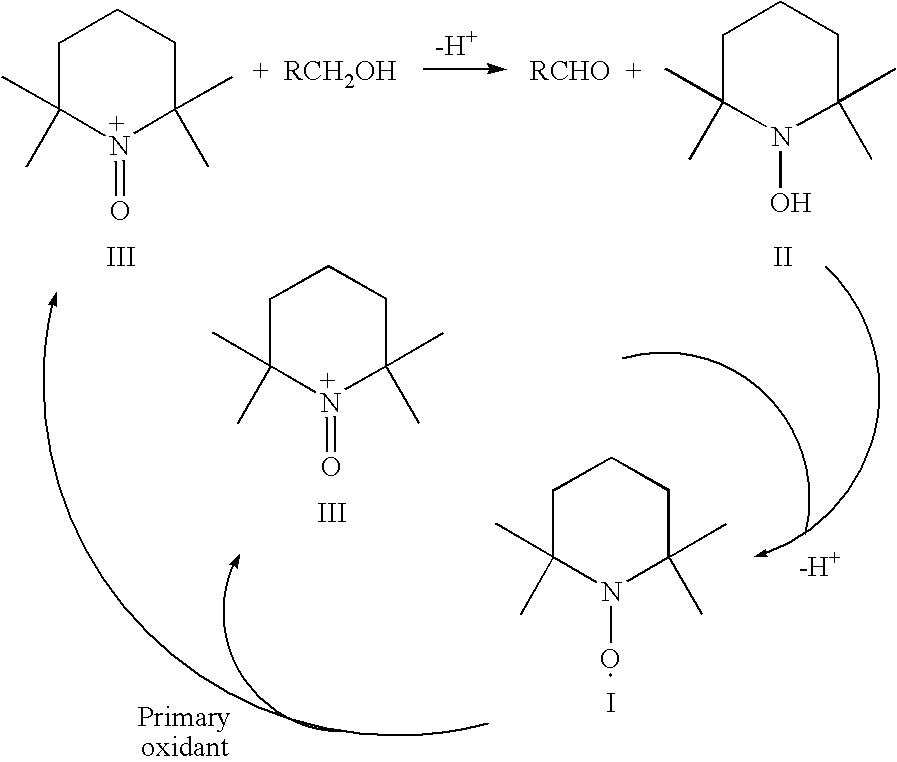
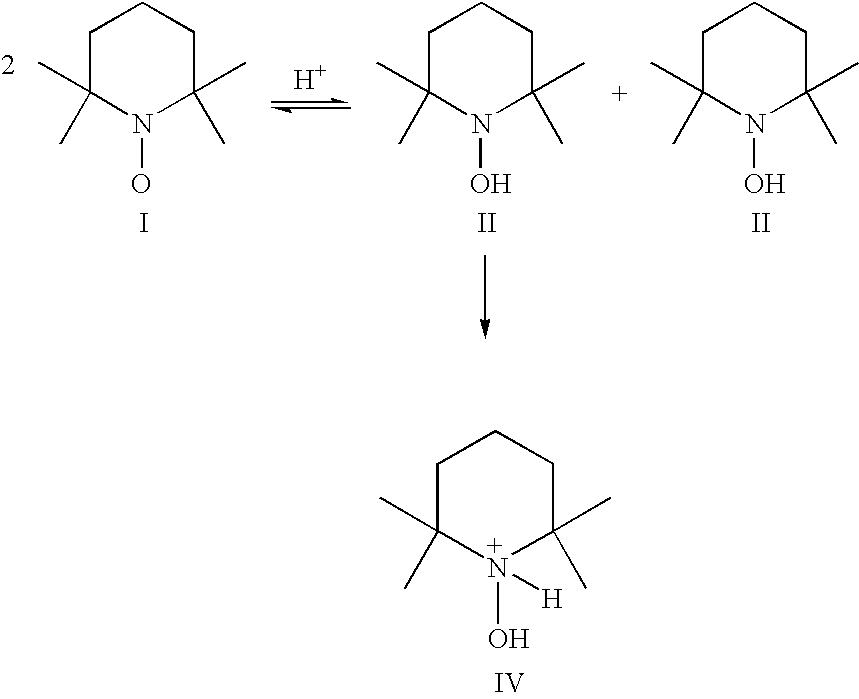
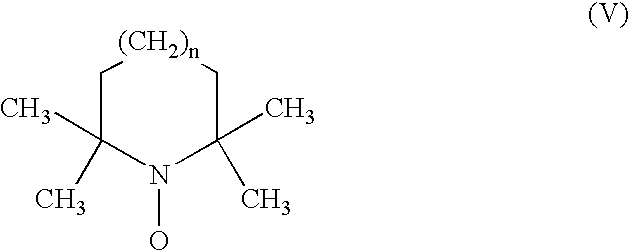
Process for the separation of organic nitrosonium and/or hydroxylamine compounds by means of cation exchange resins and recovery and oxidation processes based thereon
a technology of organic nitrosonium and hydroxylamine, which is applied in the field of organic nitrosonium and/or hydroxylamine compounds by means of cation exchange resins and recovery and oxidation processes based thereon, can solve the problems of high cost, inability to meet the requirements of economic processes, and often lost nitroxy compounds, etc., to achieve easy purification, avoid the use of organic solvents, and facilitate the recycling of various starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0122] To 1 L of a solution containing 4 g TEMPO, 15 ml of concentrated hydrochloric acid (37%) was added. The mixture was allowed to react for 30 minutes at room temperature. From this solution the absorbance at λ=430 mm (characteristic for TEMPO) and at λ=480 mm (characteristic for the corresponding nitrosonium salt) were measured and compared with the absorbance of the original solution. From the spectra it appeared that the mixture was disproportionated. 600 ml of the solution containing the protonated hydroxylamine (15 mmol) and nitrosnonium ion (15 mmol) was poured over a column containing 30 g cation exchanger (Dowex 50WX8). In the eluate the extinction of both TEMPO and its nitrosonium salt were measured (see Table 1). As can be seen, the eluted liquid contains only a limited amount of nitrosonium ion. To release the charged intermediates, the ion exchanger was washed with about 600 ml 1M hydrochloric acid. From this solution the absorbance at λ=430 and at 480 nm were measur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total carbon number | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com