Methods of diagnosing and treating pre-eclampsia or eclampsia
a technology of eclampsia and edema, which is applied in the direction of dna/rna fragmentation, depsipeptides, peptide/protein ingredients, etc., can solve the problems of cerebral edema and seizures seen in eclampsia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
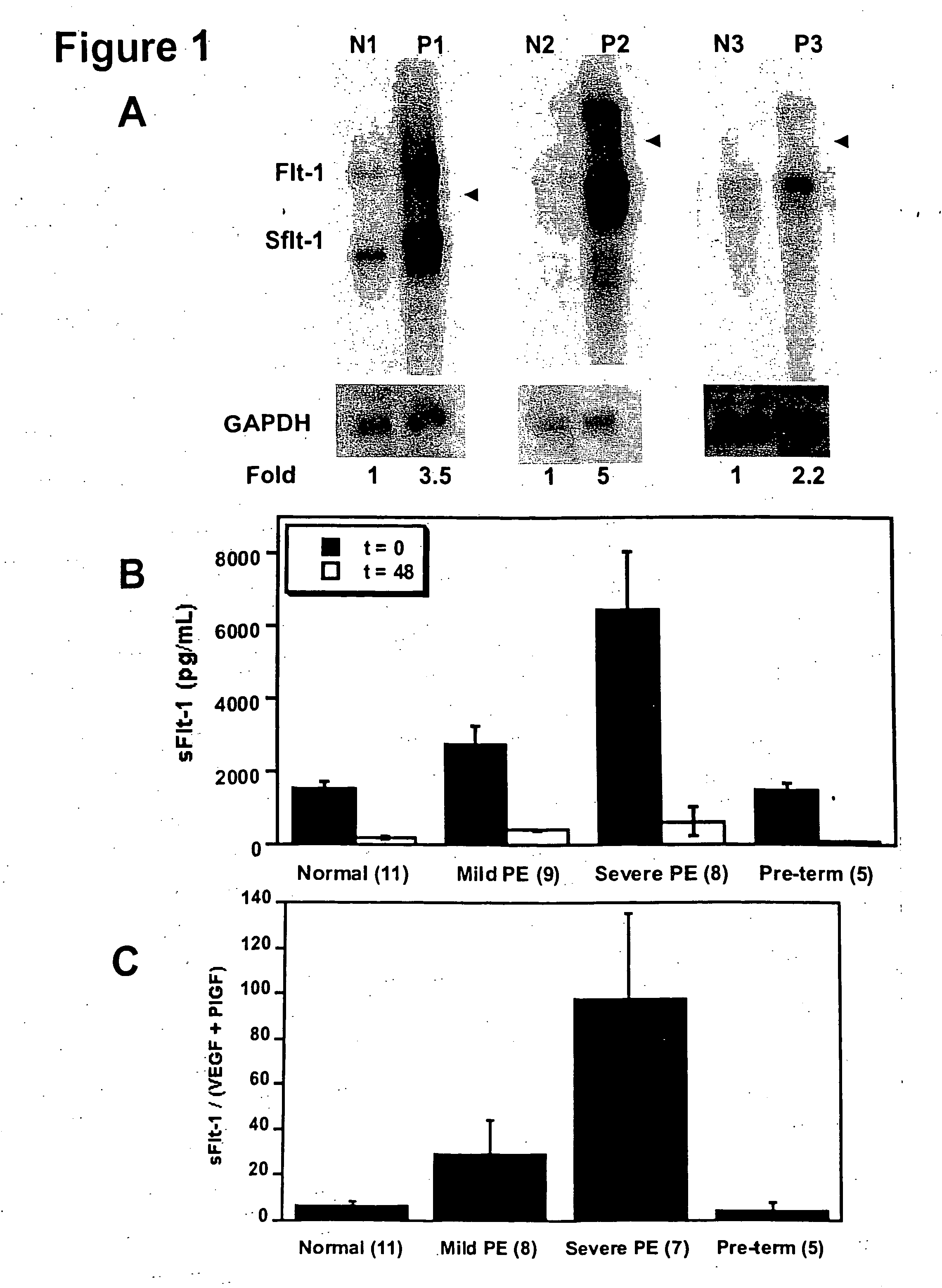
[0098] Increased Levels of sFlt-1 mRNA and Protein in Pregnant Women with Pre-eclampsia.
[0099] In an attempt to identify novel secreted factors playing a pathologic role in pre-eclampsia, we performed gene expression profiling of placental tissue from women with and without pre-eclampsia using Affymetrix U95A microarray chips. We found that the gene for sFlt-1 was upregulated in women with pre-eclampsia.
[0100] In order to confirm the upregulation of sFlt-1 in pre-eclampsia, we performed Northern blots to analyze the placental sFlt-1 mRNA levels (FIG. 1A) and ELISA assays to measure serum protein levels of sFlt-1 (FIG. 1B) in pre-eclamptic pregnant women as compared with normotensive pregnant women. Pre-eclampsia was defined as (1) a systolic blood pressure (BP)>140 mmHg and a diastolic BP>90 mmHg after 20 weeks gestation, (2) new onset proteinuria (1+ by dipstik on urinanalysis, >300 mg of protein in a 24 hour urine collection, or random urine protein / creatinine ratio>0.3, and (3)...
example 2
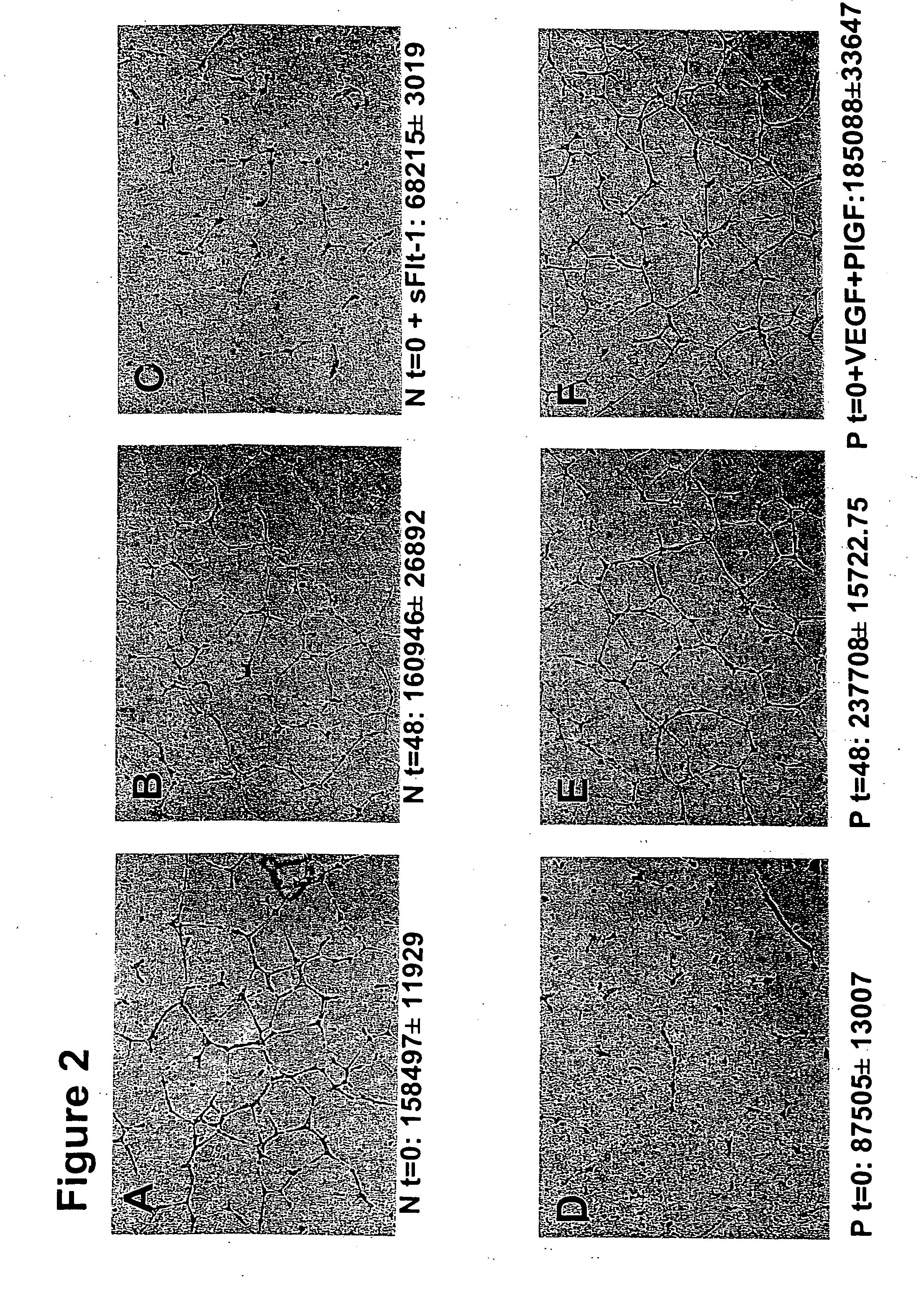
[0103] Serum from Women with Pre-eclampsia Inhibits Angiogenesis in an in vitro Endothelial Tube Assay.
[0104] We hypothesized that excess circulating sFlt-1 in patients with pre-eclampsia causes endothelial dysfunction and leads to an anti-angiogenic state. To address this, we used an endothelial tube assay as an in vitro model of angiogenesis. Growth factor reduced Matrigel (7 mg / mL, Collaborative Biomedical Products, Bedford, Mass.) was placed in wells (100 μl / well) of a pre-chilled 48-well cell culture plate and incubated at 37° C. for 25-30 minutes to allow polymerization. Human umbilical vein endothelial cells (30,000+ in 300 μl of endothelial basal medium with no serum, Clonetics, Walkersville, Md.) at passages 3-5 were treated with 10% patient serum, plated onto the Matrigel coated wells, and incubated at 37° C. for 12-16 hours. Tube formation was then assessed through an inverted phase contrast microscope at 4× (Nikon Corporation, Tokyo, Japan) and quantitatively analyzed (...
example 3
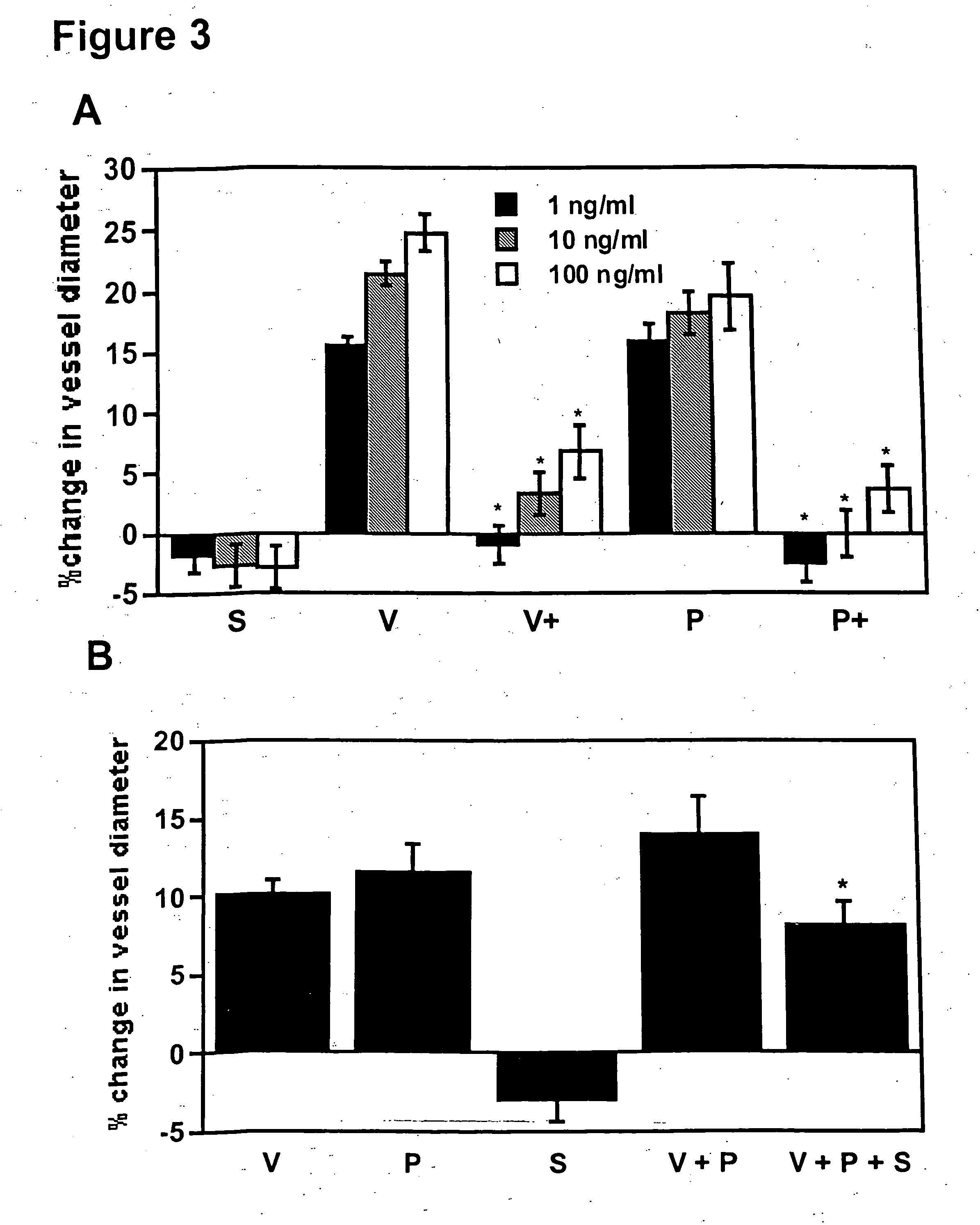
[0106] sFlt-1 Inhibits VEGF and PlGF Induced Vasodilation of Renal Microvessels.
[0107] The causative role of sFlt-1 in vasoconstriction was determined using an in vitro microvascular reactivity experiment. Microvascular reactivity experiments were done as described previously using rat renal microvessels (Sato et al., J. Surg. Res., 90:138-143, 2000). Kidney artery microvessels (70-170 μm internal diameter) were dissected from rat kidneys using a 10× to 60× dissecting microscope (Olympus Optical, Tokyo, Japan). Microvessels were placed in an isolated microvessel chamber, cannulated with dual glass micropipettes measuring 30-60 μm in diameter, and secured with a 10-0 nylon monofilament suture (Ethicon, Somerville, N.J.). Oxygenated (95% oxygen and 5% carbon dioxide) Krebs' buffer solution warmed to 37° C. was continuously circulated through the vessel chamber and a reservoir containing a total of 100 ml of the solution. The vessels were pressurized to 40 mmHg in a no-flow state usin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com