Method of generating chemical compounds having desired properties
a chemical compound and desired technology, applied in the field of chemical entity generation, can solve the problems of lead compound identification, limited search for lead compounds, and conventional approach dependence on the availability, size, and structural diversity of chemical libraries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Generation of Lead Thrombin Inhibitor
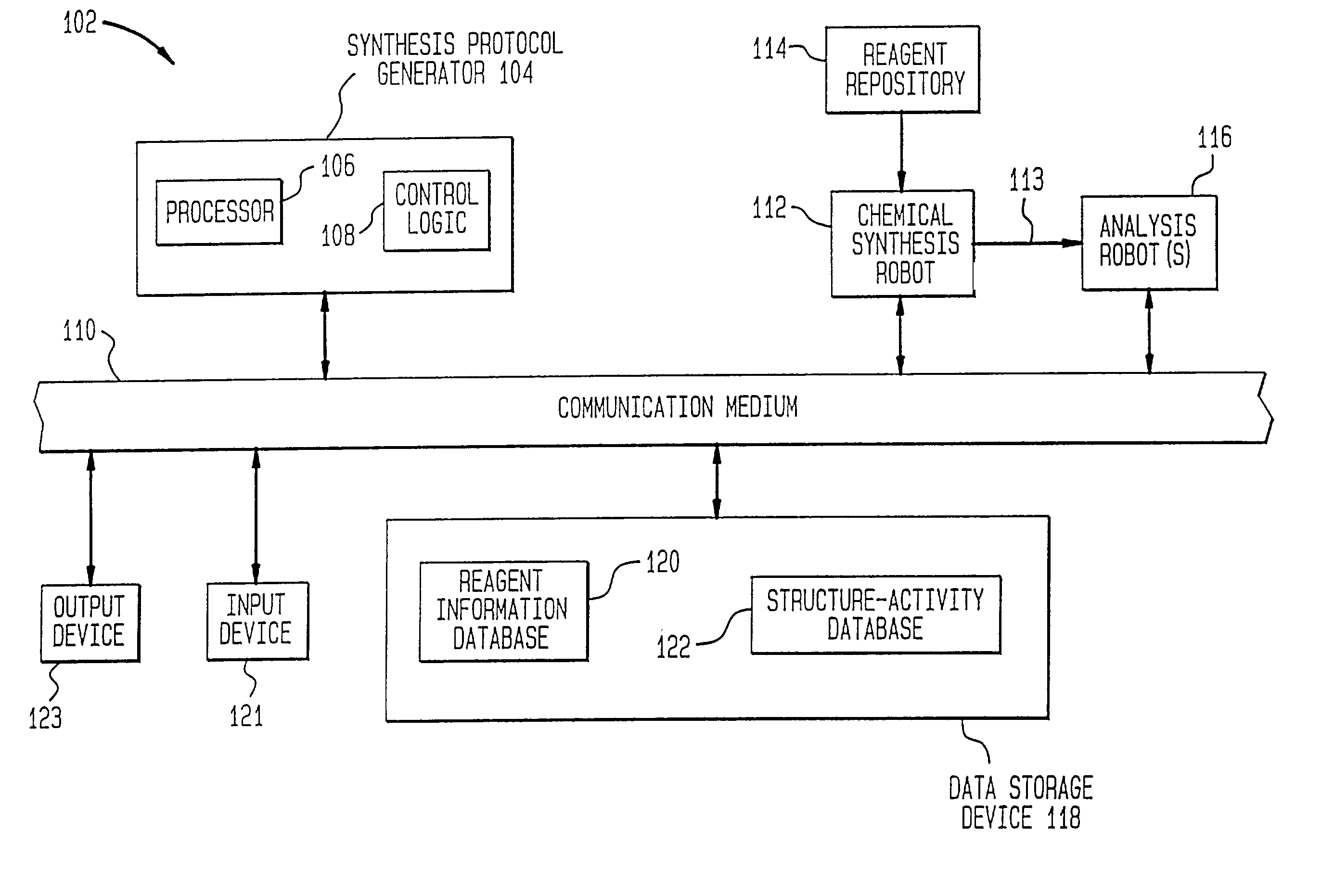
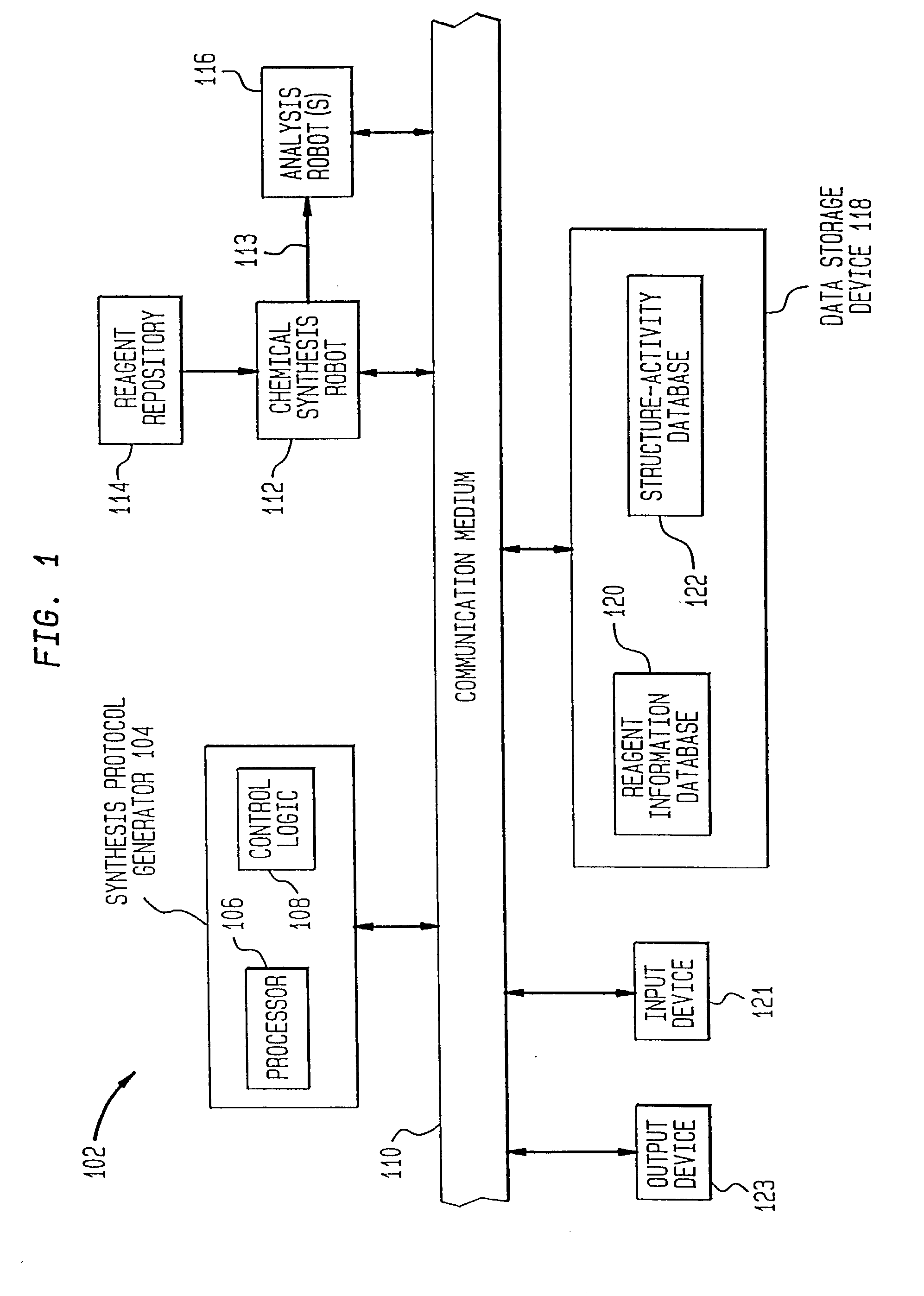
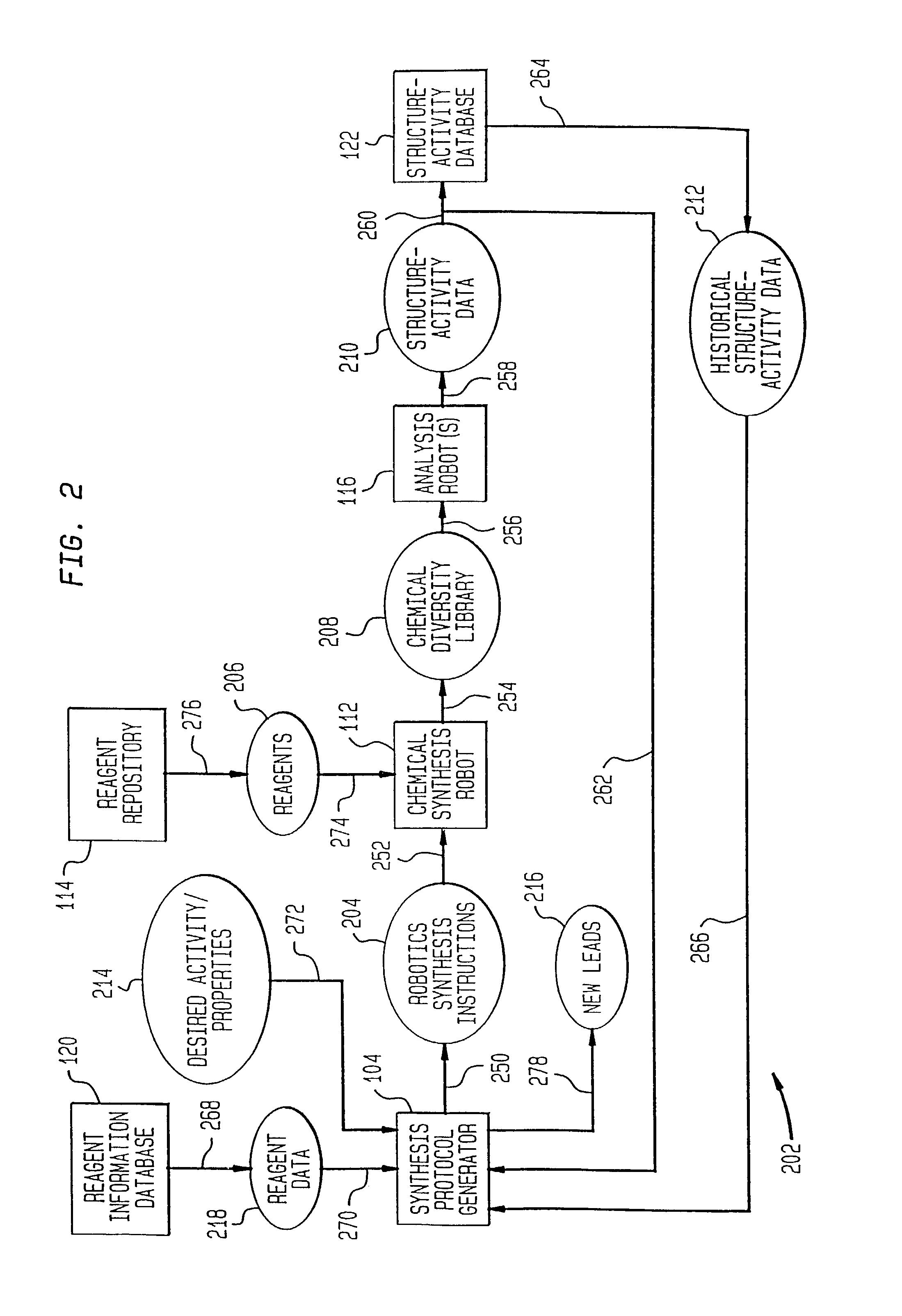
[0128] One example of the present invention is directed towards the generation and analysis of libraries of thrombin inhibitors. This example shall now be discussed.
[0129] Thrombin is a serine protease involved in both the blood coagulation cascade and platelet activation. When the circulatory system is injured, a cascade of reactions is initiated which leads to the production of thrombin. Thrombin catalyzes the conversion of fibrinogen to fibrin, which forms polymers, and the activation of factor XIII, which catalyzes fibrin crosslinking leading to the formation of fibrin clots. Thrombin also activates the thrombin receptor, which together with other signals induces platelet aggregation, adhesion and activation, and the formation of haemostatic plugs. Aberrant activation or regulation of the coagulation cascade is a major cause of morbidity and mortality in numerous diseases of the cardiovascular system and their associated surgical treatment. C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure-activity | aaaaa | aaaaa |
| structure-activity data | aaaaa | aaaaa |
| chemical compounds | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com