Endothelial cell growth factor, methods of isolation and expression
a growth factor and endothelial cell technology, applied in the field of growth factor, can solve the problems of loss of biological activity, no highly suitable mitogenic factor exists for this type of application, and no mitogenic activity has been purified or characterized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Culture of Follicular Cells and Media Collection
[0238] Primary cultures of bovine pituitary FC were established as previously described (20, 41). In one embodiment in the culturing the 20% fetal bovine serum in reference 20 was reduced to 10%. Concentrations of 5 to 20% should be effective. Also no DNAase is used. All other components are the same. At confluency, cells were passaged into large scale tissue culture plates in the presence of low glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine and antibiotics. Shortly after reaching confluency the cultures were extensively washed with PBS in order to remove serum components. The cells were then incubated in a serum-free medium consisting of DMEM plus transferrin (10 .mu.l / ml), insulin (5 .mu.g / ml), selenium (10.sup.-8 M), 2 mM glutamine and antibiotics. After three or four days, the medium was collected and replaced with fresh serum free medium. The collected medium was centrif...
example 2
Concentration of Conditioned Medium
[0239] Four to six liter batches of conditioned medium (CM) were subjected to ammonium sulfate precipitation. Ammonium sulfate (500 g / L) was added under constant stirring, until the salt was completely in solution. After 8-12 hours in the cold room, the material was centrifuged (20,000 .times.g, 45 min at 4.degree. C.). The supernatant was discarded and the pellet was resuspended with 10 mM Tris / Cl, pH 7.2, 50 mM NaCl and dialyzed at 4.degree. C. against the same buffer for 8-12 h. The final volume was 50-60 fold less than the original.
[0240] Alternatively, the CM is concentrated using ultrafiltration using an Amecon stir cell (2.5 liter unit) using a membrane having a molecular weight cut off of 10,000 daltons with similar results.
example 3
Heparin-Sepharose Affinity Chromatography
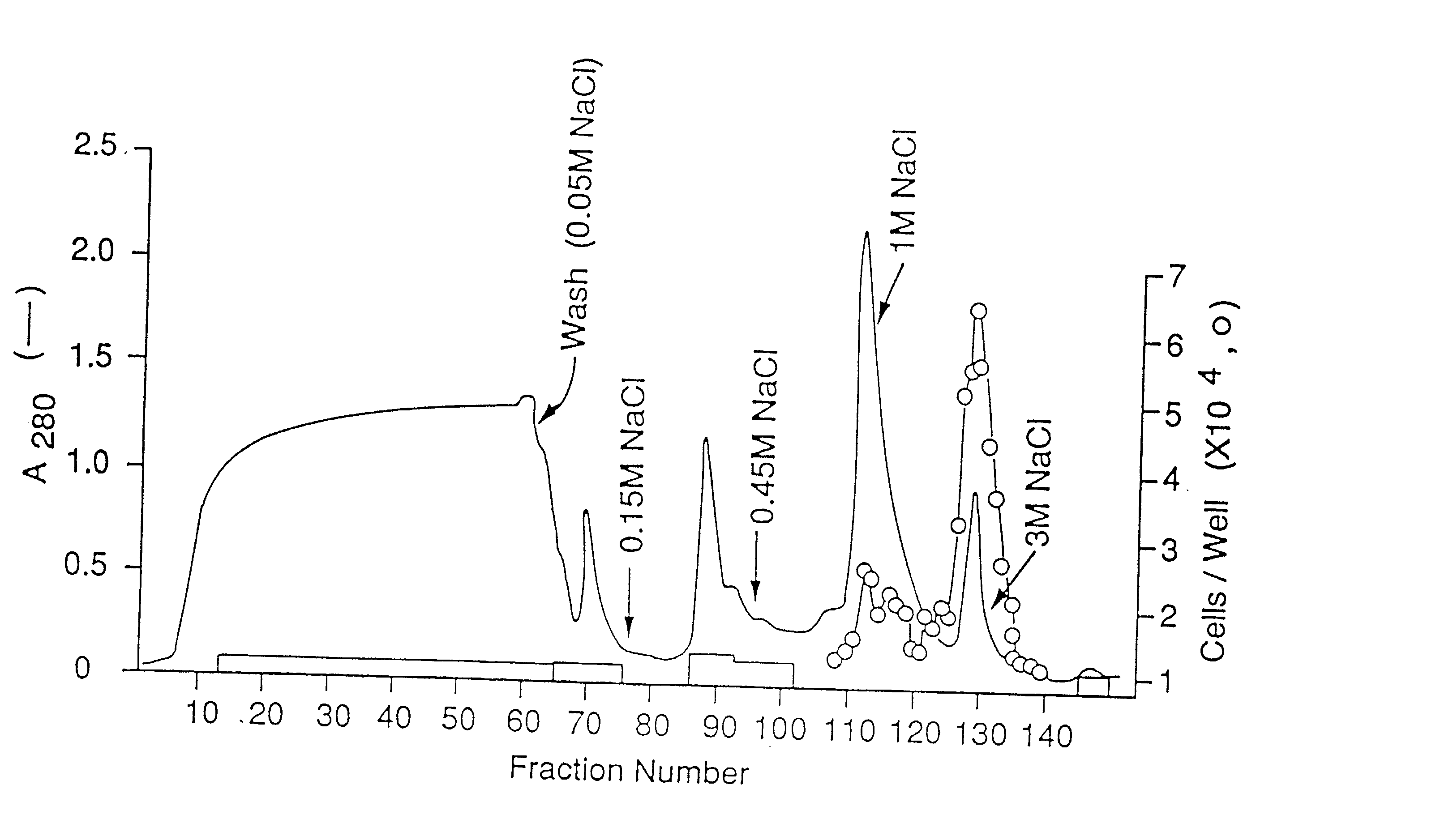
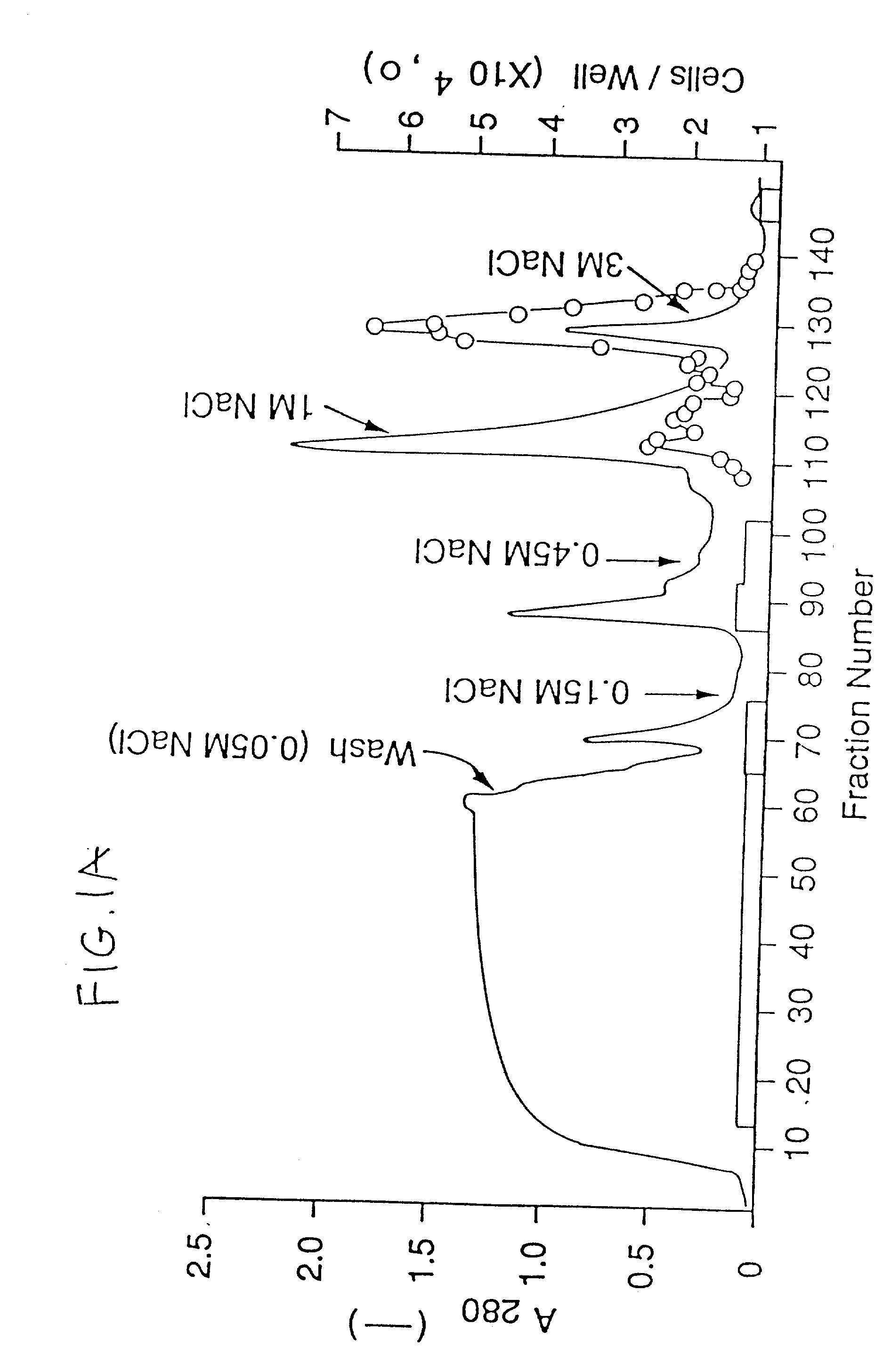
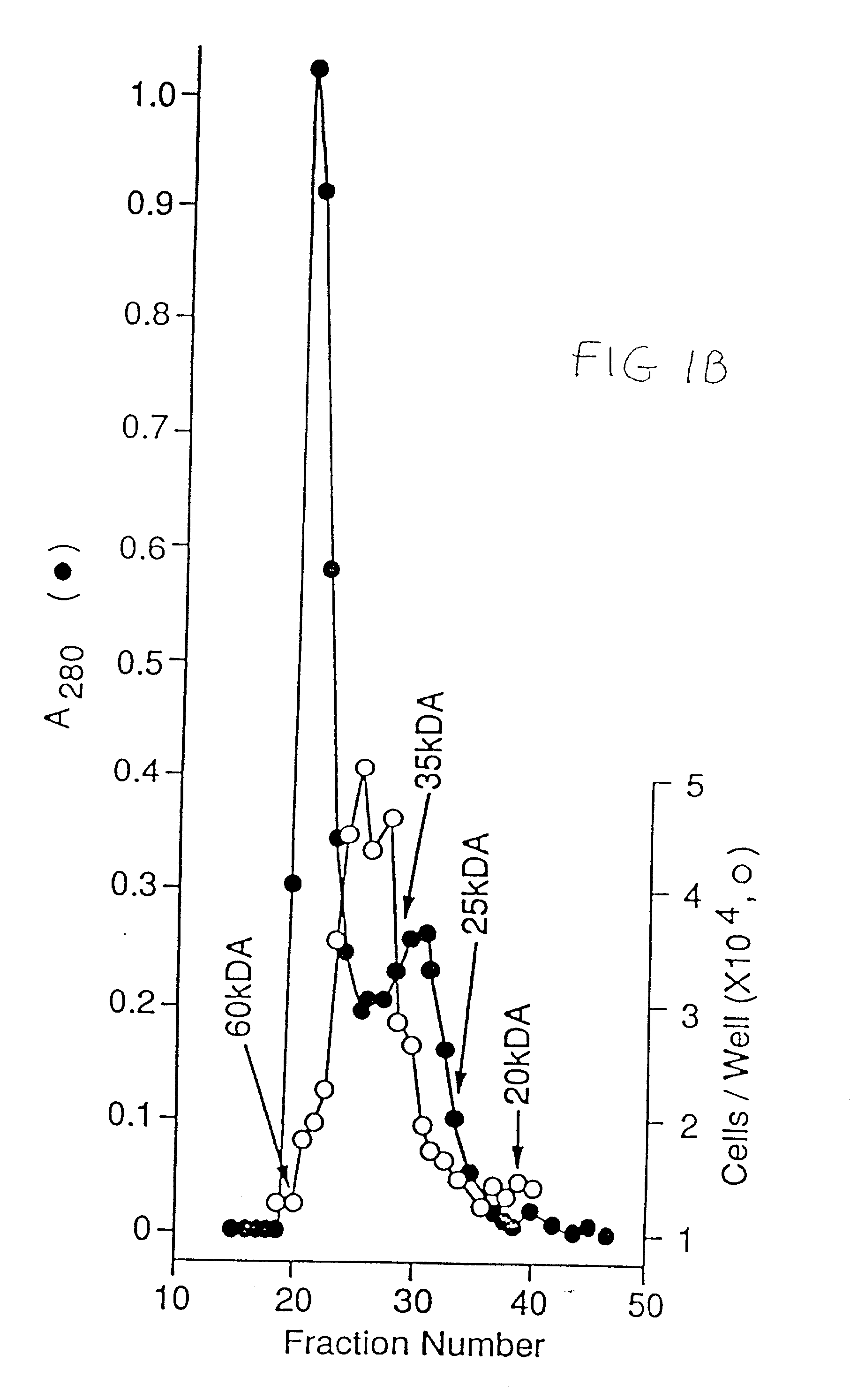
[0241] The concentrated CM was applied to a H-S column (14) (10 ml) preequilibrated with 10 mM Tris / Cl, pH 7.2, 50 mM NaCl. The column was then washed with the same buffer until the absorbance at 280 nm was negligible and then eluted stepwise with 10 mM Tris / Cl pH 7.2 containing 0.15, 0.9 nd 3 M NaCl. The flow rate was 1.5 ml / min. Fractions of 1.5 ml were collected and aliquots, diluted with 0.2% gelatin in PBS, were tested for mitogenic activity on endothelial cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com