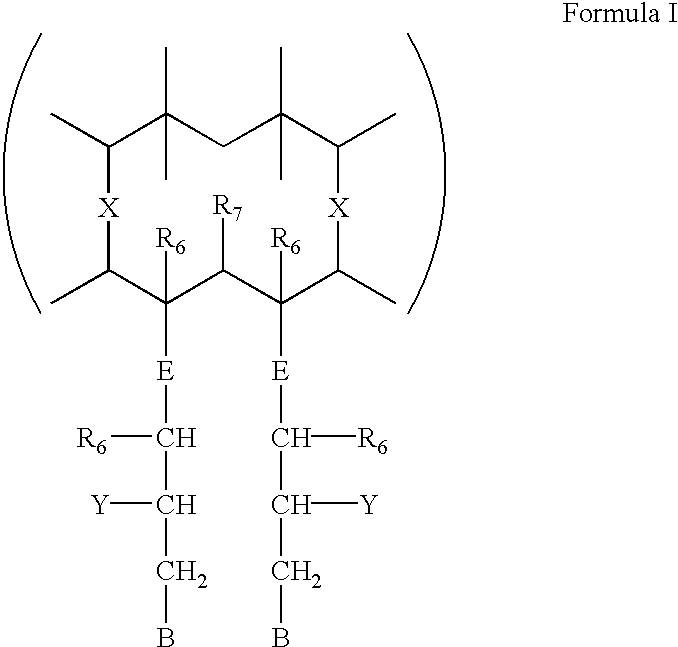
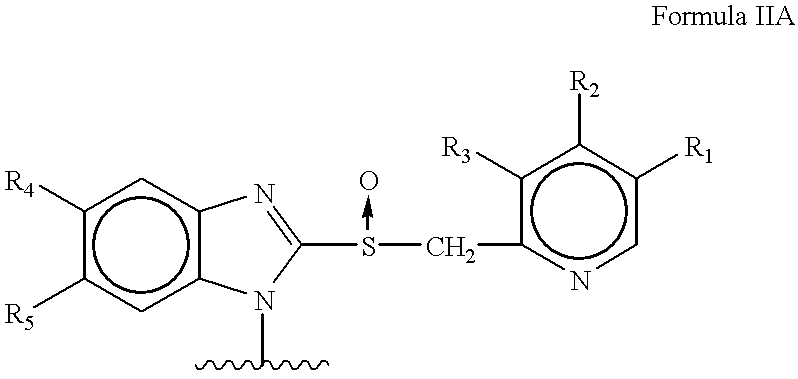
Orally administrable acid stable antiulcer benzimidazole derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0061] The copolymer (5.0 g) prepared using glycidyl methacrylate and acrylamide was mixed with omeprazole (1.25 g) dissolved in aqueous medium at pH 9.8. The reaction mixture was stirred at 30.degree. C. for 18 hours. The product was filtered washed with water (100 ml.times.5) and dried under vacuum at 45.degree. C. for 12 hours to obtain 5.92 g of the polymer-substituted omeprazole.
example 3
[0062] The copolymer (5.0 g) prepared using glycidyl methacrylate and glycol dimethacrylate was mixed with omeprazole (1.25 g) dissolved in aqueous madium at pH 10.4. The reaction mixture was stirred at 30.degree. C. for 18 hours. The product was filtered washed with water (100 ml.times.5) and dried under vacuum at 45.degree. C. for 12 hours to obtain 6.05 g of the polymer-substituted omeprazole.
example 4
[0063] The procedure of Example 2 was followed using omeprazole (2.5 g) and pH 10.2 instead of omeprazole (1.25 g) and pH 9.8 to obtain 6.45 g of the polymer-substituted omeprazole.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com