Curcumenol glucoside compound and its preparation method and uses
A technology of curcumol and compounds, which is applied in the field of new curcumol glycoside compounds and their preparation and application, can solve the problems of low water solubility and difficult preparation, and achieve high solubility, enhanced water solubility, and improved bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
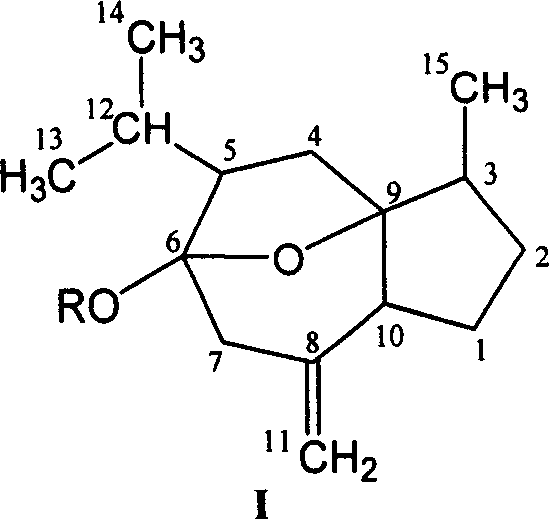
[0014] Preparation of Curcumol Glycosides
[0015] Curcuma alcohol is the most abundant component in curcuma oil. After further separation and purification of commercially available curcuma, curcumol with a purity of more than 95% can be obtained, which is used as the raw material for glycosylation modification of curcumol structure in the invention patent. .
[0016] (1). Preparation of curcumitol (hydroxyl on C-6 position) glycoside derivatives
[0017] The 6-hydroxyl group in the curcumitol molecule can be regarded as the product of 6-carbonyl monoketal. Since the 6-carbon is connected with a hydroxyl group and an oxygen bridge, due to the steric effect and the electron-donating effect of the oxygen bridge, its The stability and reactivity are slightly weaker than the general hydroxy compounds, and the reaction conditions should be carefully controlled during the reaction.
[0018] The reaction process is as follows:
[0019] Step 1: Synthesis of Bromoacetylated Sugars ...
Embodiment approach
[0032] When different saccharide compounds are used as reaction raw materials, the proportion of raw materials in the reaction should be adjusted according to the specific situation.
[0033] The following examples are only for further illustrating the present invention, rather than limiting the present invention.
Embodiment 1
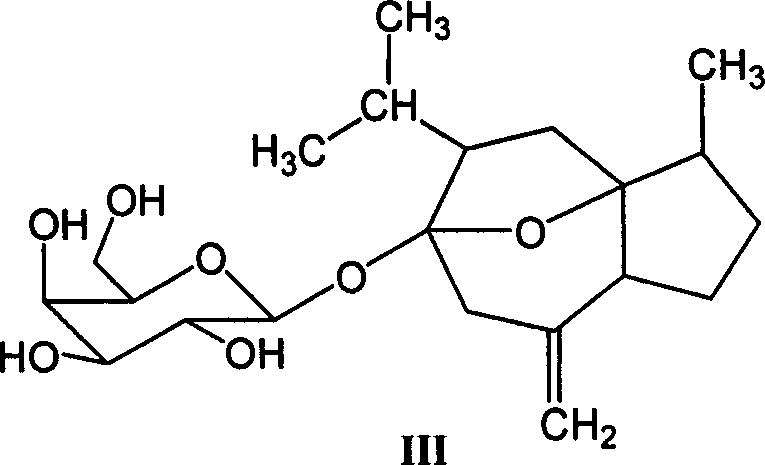
[0034] Embodiment 1: the preparation of curcumitol-6-O-β-D-glucopyranoside (II);
[0035]
[0036] ① Add 216 g (200 mL, 2.12 mol) of acetic anhydride to a 500 mL four-neck round-bottomed flask equipped with a mechanical stirrer, a thermometer, and a dropping funnel under ice-bath cooling. After cooling to 0 °C, slowly add 1.2 mL ( 70% to 72%, 0.02mol) perchloric acid. After dripping, the temperature was controlled between 30 and 40° C., and 50 g (0.28 mol) of dry glucose powder was added in batches. After the reaction was completed, it was cooled to 0°C, 15.5 g (0.5 mol) of red phosphorus was added, 90.5 g (29 mL, 1.13 mol) of liquid bromine was added dropwise within 4 h, 150 mL of chloroform was added after the reaction was completed, and the unreacted red phosphorus was removed by filtration , the filtrate was washed three times with 100 mL of ice water, and then washed with saturated sodium bicarbonate until the pH was about 7, and the organic layer was dried with molec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com