Production method of recombined human alpha1-thymus peptide and preparation thereof
A production method and technology of thymosin, applied in the production method and preparation field of recombinant human α1-thymosin, can solve the problems of high price, difficult synthesis, unbearable for ordinary patients, etc., and achieve the effect of low cost and guaranteed consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
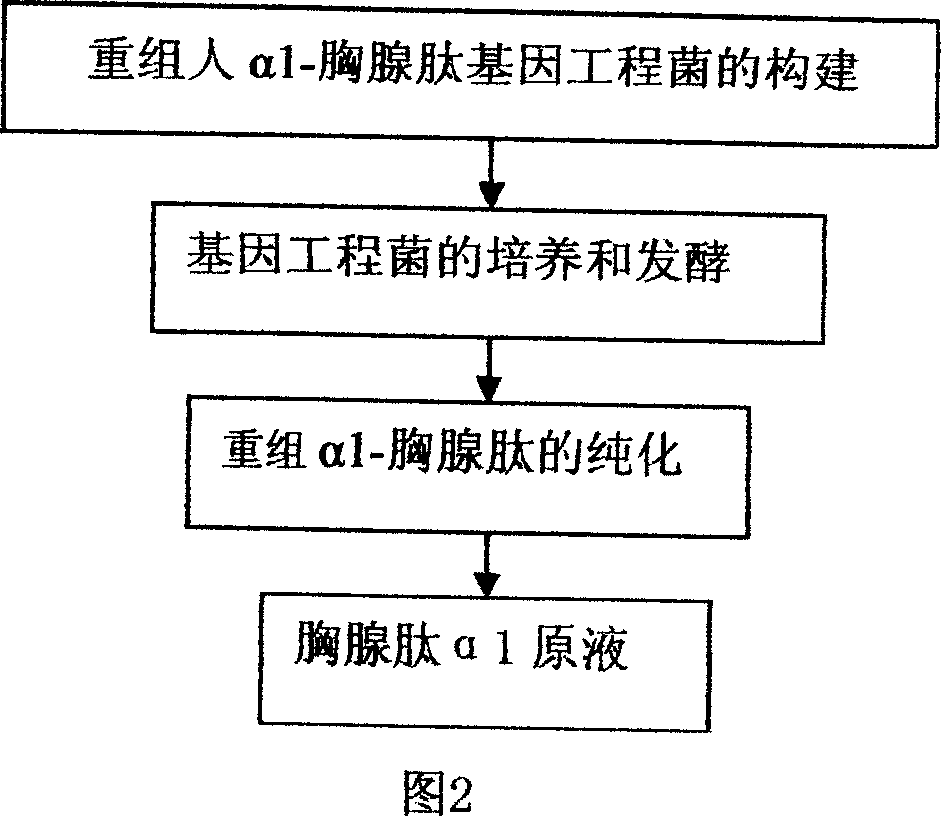
[0031] Construction of genetically engineered bacteria highly expressing human α1-thymosin:
[0032] 1. Acquisition of the full-length base sequence of human α1-thymosin gene: According to the amino acid sequence of human α1-thymosin, the following DNA fragments were synthesized:
[0033] 5'-CCT CTA GAA ATA ATT TTG TTT AAC TTT AAG AAG GAG ATA TACATA TGT CTG GAT CAG GTC ATC ATC ATC ATC ATC ATT CTT CTG GTA CCGATG ACG ACG ACA AGA GCG ATG CCG CCG TGG ATA CCA GCA GCG AAATTA CCA CCA AAG ATC TGA AAG AAA AAA AAG AAG TGG TGG AAG AAGCCG AAA ACT AAG ACT AGT GAA TTC AC-3'
[0034] The fragment sequentially includes SD sequence, purification tag His-tag, enterokinase restriction site, full-length human α1-thymosin gene and stop codon.
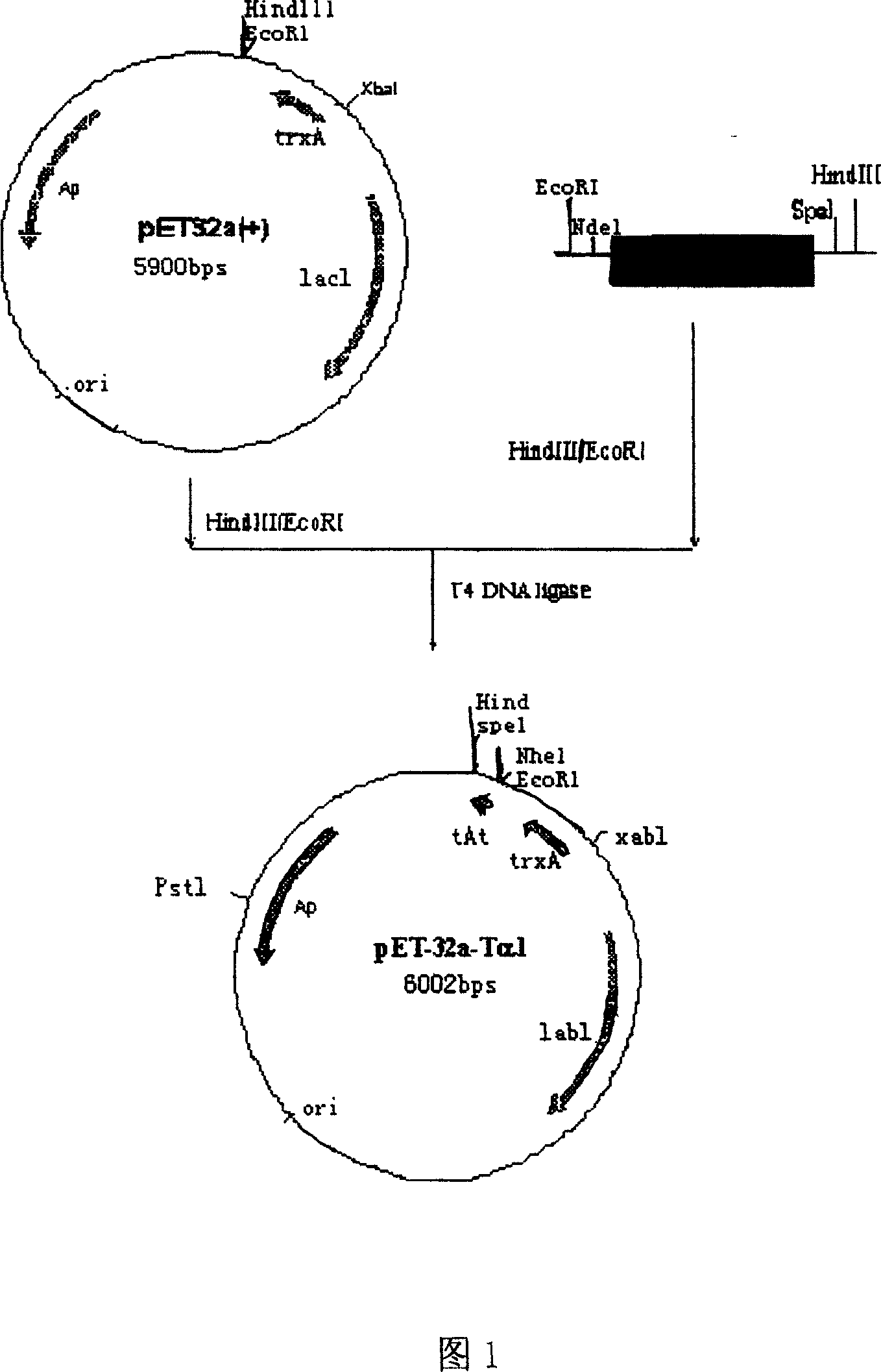
[0035] 2. Digest the fragment with XbaI / EoRI double enzymes in a 37°C water bath, recover from the gel, and connect it with the large fragment recovered from pET-32a(+) XbaI / EoRI double enzyme digestion to construct the expression vector pET-32a-Tα1 (as sho...
Embodiment 2
[0038] Fermentation of genetically engineered bacteria
[0039] 1. Take and inoculate the constructed genetically engineered bacteria into 100ml LB medium (containing 100ug / ml ampicillin), and culture in a shake flask at 37°C for 12-14h; absorb the bacterial liquid according to 10% inoculum size and add it to 500ml LB medium , 37°C, 230r / min shake flask culture for 6h, complete the preparation of seed solution;
[0040] 2. In a 30-liter fermenter (Switzerland Bioengineering Company), the seed solution was added to a fermenter equipped with 12L M9+LB medium by 5% inoculum, cultivated at 37° C. at 300 r / min, and the ventilation rate was 10 L / min. Control the pH value at about 7.0, and control the dissolved oxygen above 30%. When the OD600 is about 12, start to add the feed medium. When the OD600 is about 18, add IPTG induction, and the final concentration is 0.8mmol / L , control the dissolved oxygen curve and pH to be basically stable but slightly decreased, and the bacteria wer...
Embodiment 3
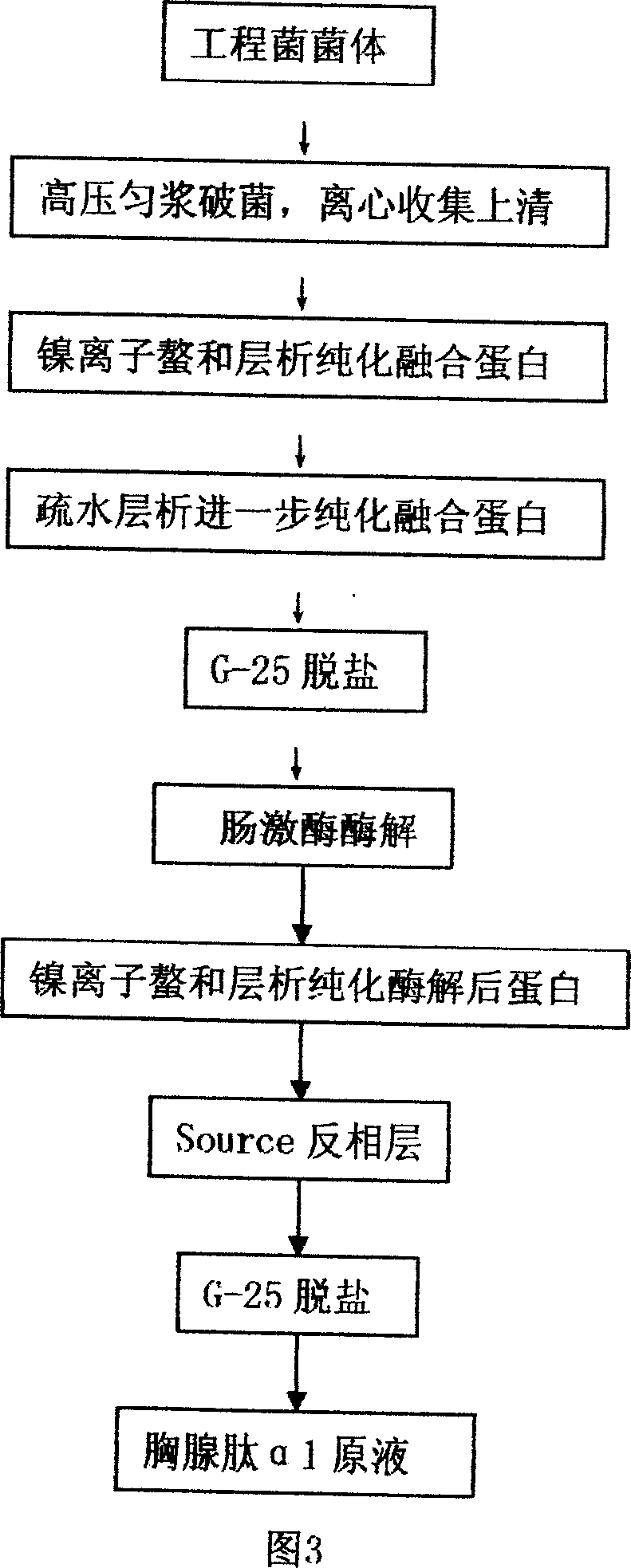
[0042] Purification of recombinant human α1-thymosin (see Figure 2)
[0043] 1. Collect the bacteria, centrifuge at 6000-7000g in continuous flow or in batches, and the centrifugation time for each batch is 8-10 minutes; use the bacteria-breaking buffer [30mmol / L imidazole-200mmol / L NaCl-20mmol / L PB (pH8 .0)] suspending, carrying out high-pressure homogenization to destroy bacteria, the operating pressure adopted during the destruction of bacteria is 70Mpa~80Mpa, and centrifuging to remove impurities after the completion of microscopic inspection of bacteria;
[0044] 2. Prepare a metal nickel ion chelating affinity chromatography column, take Chelating Sepharose FF 180mL (Amersham Biosciences company) to pack the column, wash 3 column volumes with 50mmol / L NiSO4 solution and hang Ni 2+ ; The above-mentioned centrifuged supernatant (or supernatant concentrated by ultrafiltration) is loaded, equilibrated with a buffer [30mmol / L imidazole-200mmol / L NaCl-20mmol / L PB (pH8.0)] unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com