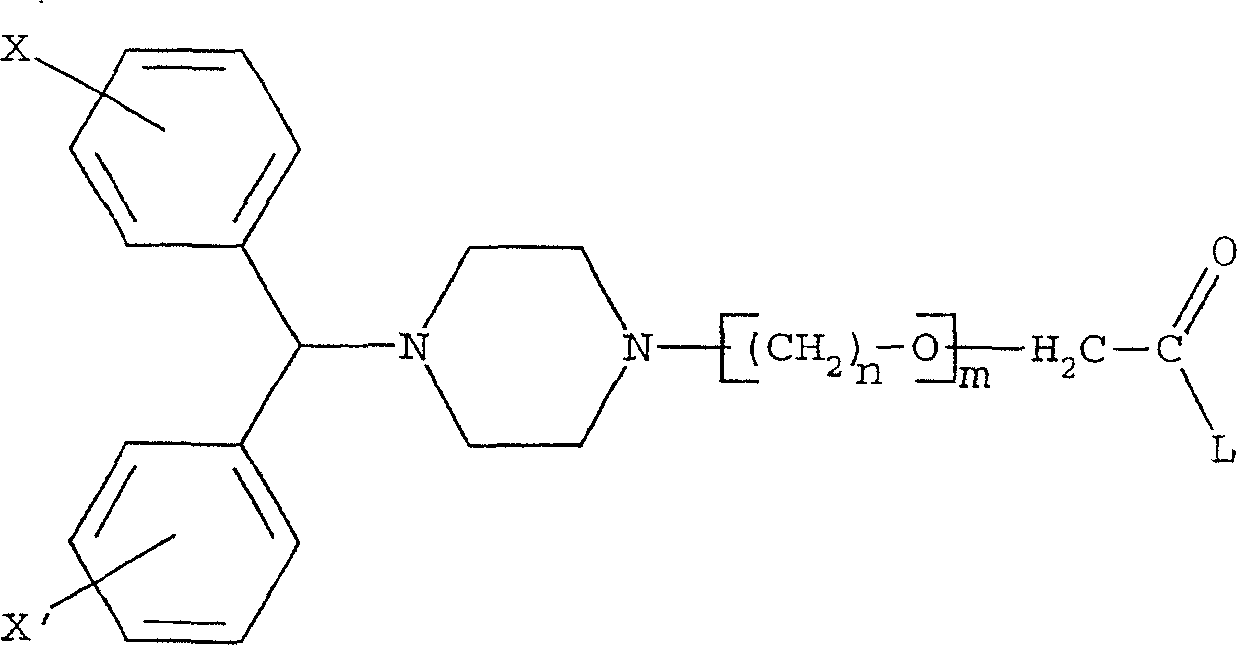
Pharmaceutical composition of piperazine derivatives
A composition and drug technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as microbial contamination, and achieve the effect of avoiding dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Antiseptic effect of cetirizine
[0041] Oral solutions and drops containing cetirizine are prepared. The composition is given in Table 1.
[0042] Table 1.- Cetirizine compositions
[0043] oral solution
drops
Cetirizine hydrochloride (mg)
1
10
70% sorbitol solution (mg)
450
-
Glycerin (mg)
200
250
Propylene Glycol (mg)
50
350
1
10
Banana spice (mg)
0.1754
-
Sodium acetate (mg)
4.2
10
Add to pH 5
Add to pH 5
Purified water (ml)
add to 1
add to 1
[0044] Preservative efficacy tests of antimicrobial agents were carried out according to the European Pharmacopoeia (chapter 5.1.3.). Oral solution and drop samples were incubated with bacterial and yeast suspensions Pseudomonas aeruginosa ATCC 9027, Escherichia coli ATCC 8739, Staphylococcus aureus ATCC6538, Candida albican...
Embodiment 2
[0051] Example 2. Antiseptic effect of levocetirizine
[0052] Oral solutions and drops containing levocetirizine are prepared. The composition is given in Table 4.
[0053] Table 4.- Levocetirizine compositions
[0054] oral solution
[0055] Preservative efficacy tests of antimicrobial agents were carried out according to the European Pharmacopoeia (chapter 5.1.3.). Oral solution and drop samples were incubated with bacterial and yeast suspensions Pseudomonas aeruginosa ATCC 9027, Escherichia coli ATCC 8739, Staphylococcus aureus ATC C6538, Candida albicans ATCC 10231 and A. niger ATCC 16404. The number of viable microorganisms per ml of preparation is determined under the test conditions. The results are given in Tables 5 and 6.
[0056] Table 5.- Microorganism content in inoculated samples of oral solutions
[0057] time (days)
[0058] Table 6. Microbial content in the inoculated samples of the drops
[0059] time (days)
[0060] I...
Embodiment 3
[0061] Example 3. Antimicrobial preservative effect of parabens on aqueous solutions of cetirizine fruit
[0062] Oral solutions and drops containing cetirizine were prepared according to Example 1, but also containing a mixture of parabens (methylparaben / propylparaben in a weight ratio of 9 / 1) . The total amount of parabens was 0.15 mg / ml, 0.45 mg / ml, 0.75 mg / ml and 1.05 mg / ml. The antimicrobial preservation effect of these solutions and drops was determined according to the European Pharmacopoeia (Chapter 5.1.3.). The test results are given in Tables 7-14.
[0063] Table 7. Microorganism content in inoculated samples of oral solution containing 0.15 mg / ml paraben
[0064] time (days)
Pseudomonas aeruginosa fake sheet
Escherichia coli
bacillus
golden yellow
white rosary
Aspergillus niger
Inoculum
5.5×10 5
4.6×10 5
4.0×10 5
3.7×10 5
2.3×10 6
0
5.1×1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com