Improved antimicrobial peptides
An antimicrobial peptide and antimicrobial technology, applied in the field of antimicrobial peptides, can solve the problems of reducing effective use and increasing resistance problems, and achieve the effect of increasing possibility, enhancing effect and preventing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
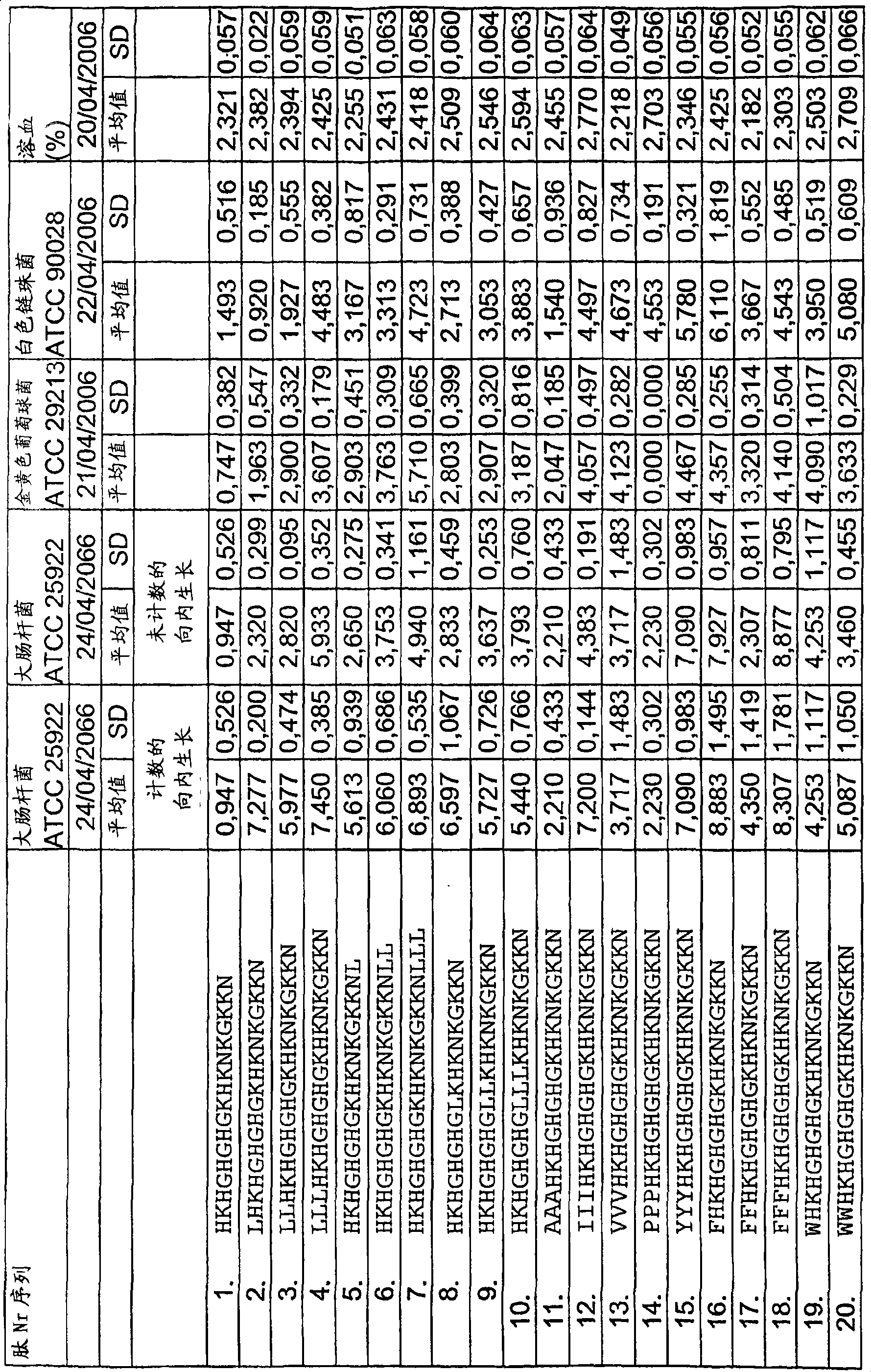
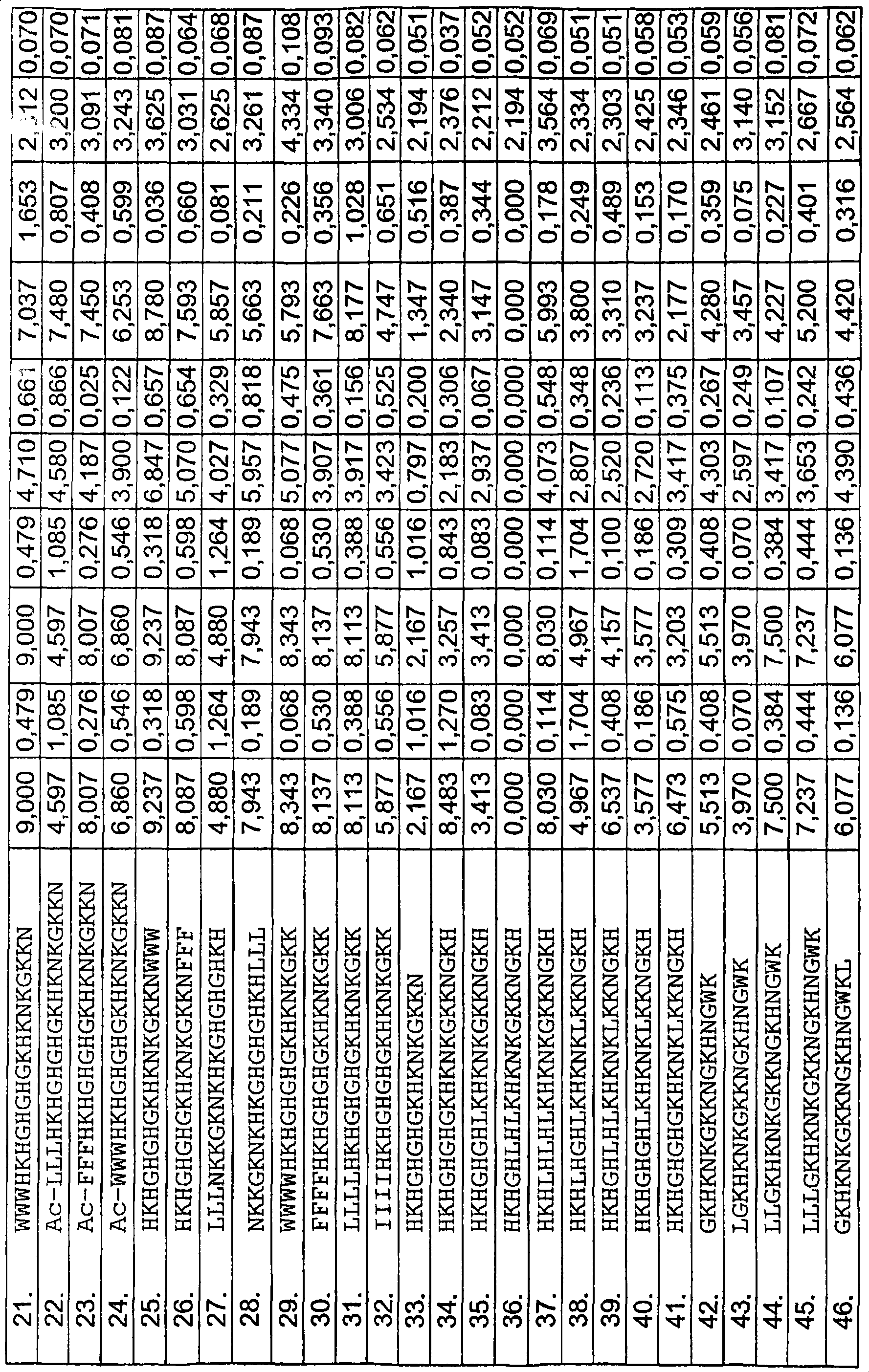
[0097] Radial diffusion assay
[0098] It was generally performed as described earlier (Lehrer, RI, Rosenman, M., Harwig, SS, Jackson, R. & Eisenhauer, P. (1991) Ultrasensitive assays for endogenous antimicrobial polypeptides, J Immunol Methods. 137, 167-73.) Radial Diffusion Assay (RDA). The results are shown in Tables 1a-e.
[0099] In short, the bacteria (E. coli, Staphylococcus aureus) in 10 ml of full-strength (3% w / v) tryptic soy broth (TSB) (Becton-Dickinson, Cockeysville, MD) ) Or fungus (Candida albicans) cultured to midlogarithmic phase. Use 10mM Tris, pH7.4 to wash the microorganisms once. To 5ml of the underlay agarose gel (from 0.03% (w / v) TSB, 1% (w / v) low electroosmotic (Low-EEO) agarose (Sigma, St Louise MO) and 0.02% ( v / v) the final concentration of Tween20 (Sigma) composition) by adding 4x10 6 Bacterial cfu or 1x10 5 Fungal cfu. Pour the bottom layer into a petri dish with a diameter of 85 mm. After the agarose is solidified, a small hole with a diameter o...
Embodiment 2
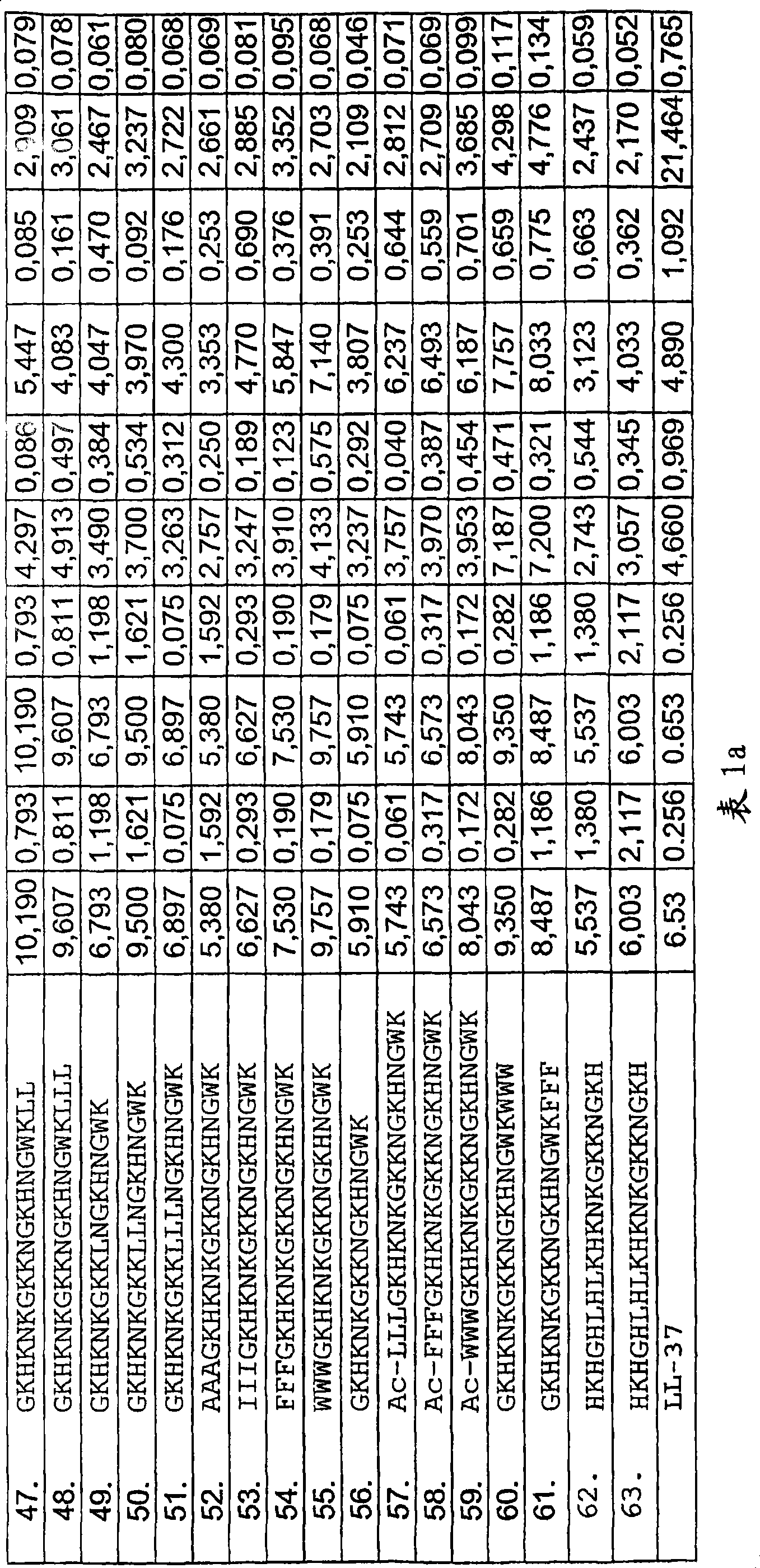
[0127] Hemolyis assay
[0128] Centrifuge the EDTA-blood at 800 g for 10 minutes, and then remove the plasma and buffy coat. The red blood cells were washed 3 times, and then resuspended in 5% PBS, pH 7.4. The cells were then incubated at 37°C for 1 hour in the presence of peptide (3-60 µM) under conditions of end-over-end rotation. 2% Triton X-100 (Sigma-Aldrich) was used as a positive control. The sample was then centrifuged at 800g for 10 minutes. The absorbance of hemoglobin released was measured at λ540 nm, and the absorbance is expressed in the figure as the percentage of hemolysis induced by Triton-X100 (Table 1a).
[0129]
[0130]
[0131]
[0132]
[0133]
[0134] Table 1b
[0135]
[0136] Table 1c
[0137]
[0138] Table 1d
[0139]
[0140] Table 1e
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com