Ocular lens material and method for producing same
A manufacturing method and glasses technology, applied in glasses/goggles, intraocular lenses, eye implants, etc., can solve problems such as failure to obtain contact lenses, achieve excellent antifouling effect, inhibit protein adsorption, and improve water retention Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] (the preparation method of the phosphorylcholine-containing compound of formula (2))
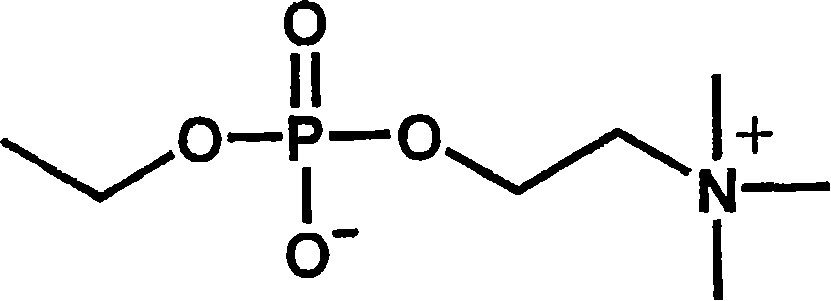
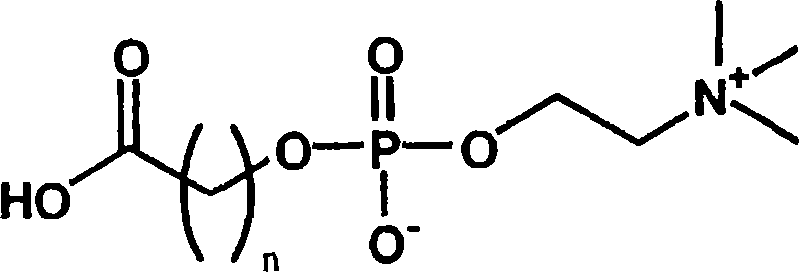
[0042] Phosphocholine groups can be prepared completely synthetically. However, the synthesis conditions are complicated, must be strictly anhydrous conditions, and the preparation cost is high.
[0043] On the other hand, phosphorylcholine can be extracted in the form of lecithin, which is a constituent of the cell membrane. In this case, 1-α-glycerophosphocholine can be obtained easily and at a lower cost by removing the fatty acid moiety by hydrolysis. The inventors of the present invention have found that a phosphorylcholine group-containing compound having a carboxyl group can be easily obtained by oxidatively cleaving the diol portion of this 1-α-glycerophosphorylcholine.
[0044] The most representative synthesis method is: oxidizing 1-α-glycerophosphorylcholine in water, acetonitrile and other solvents through sodium periodate and ruthenium trichloride to obtain the target ca...
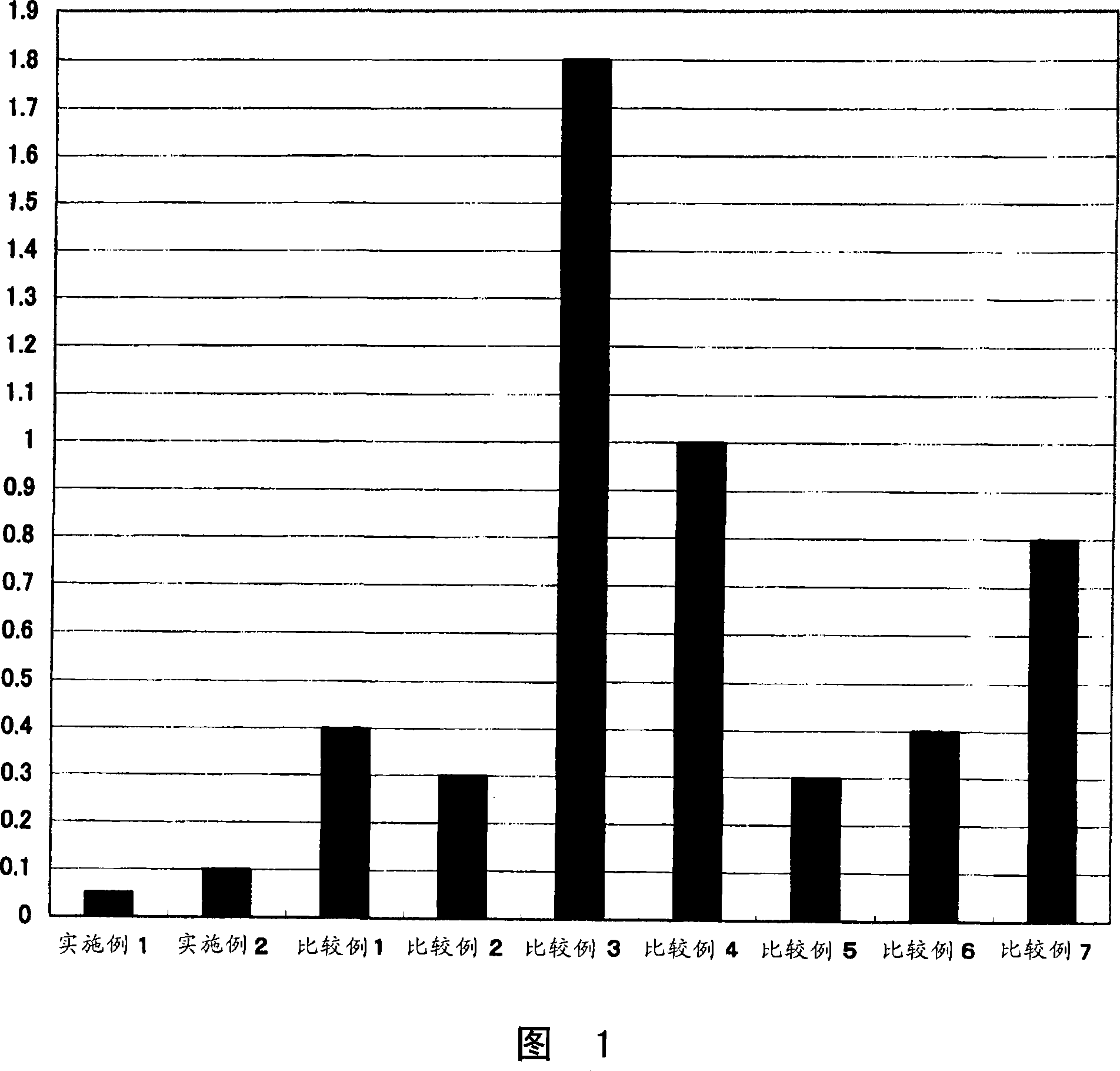
Embodiment 1
[0076] (Example 1: A contact lens in which a compound of formula (2) forms an ester bond with Polymacon (a soft contact lens Medalist manufactured by BaushLomb Co., Ltd.) via a carboxylic acid halide)
[0077] 10 mg of the compound of formula (2) was added to 2 ml of dimethylformamide, 5 mg of thionyl chloride was added, followed by stirring for 30 minutes. Soak 1 piece of Polymacon fully replaced with dimethylformamide to remove water, add 0.2ml of triethylamine, and then react for 4 hours. The reaction solution was sufficiently washed with pure water to obtain a contact lens.
Embodiment 2
[0078] "Example 2: A contact lens in which an ester bond is formed between a compound of formula (2) and Nelfilcon A (Fokas de Iris made by Cibabizion)"
[0079] Fully replace Nelfilcon A with dimethylformamide to remove water, then soak in 2ml of dimethylsulfoxide, add 10mg of the compound of formula (2), 1-ethyl-3-(3-dimethylaminopropyl) Carbodiimide hydrochloride 7 mg, then reacted for 6 hours. The reaction solution was sufficiently washed with pure water to obtain a contact lens.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com