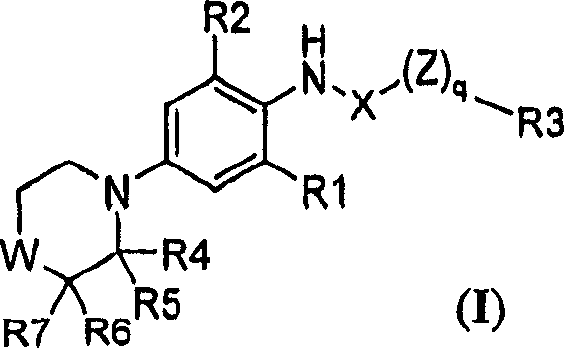
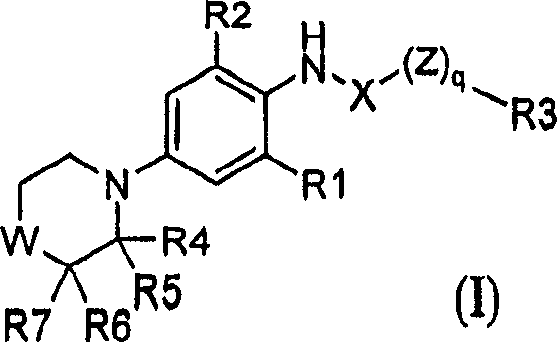
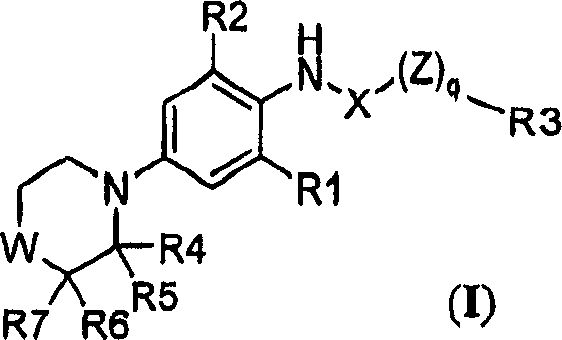
Substituted morpholine and thiomorpholine derivatives
A technology of thiomorpholine and derivatives, which is applied in the field of openers of KCNQ family potassium ion channels, and can solve problems such as reducing neuronal excitability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0540] 1a N-(2-Bromo-4-morpholin-4-yl-6-trifluoromethyl-phenyl)-2-(4-fluoro-phenyl)-acetamide.
[0541] In a sealed microwaved vial, 2-bromo-4-morpholin-4-yl-6-trifluoromethyl-aniline (0.236 g) and 4-fluorophenylacetyl chloride (0.105 mL) were dissolved in acetonitrile (5 ml) and heated to 150°C for 10 minutes. Water (25 mL) was added and the product was extracted with ethyl acetate (3 x 25 mL). The organic phase was washed with brine (50 mL), dried over magnesium sulfate, and concentrated in vacuo. The crude product was purified by flash chromatography to provide 0.027 g (9%) of the title compound as an off-white solid. LC-MS (m / z) 462 (MH + ); R =2.84, (UV, ELSD) 96%, 100%. 1 H NMR (500MHz, DMSO-d 6 ): 3.23(m, 4H), 3.62(s, 2H), 3.72(m, 4H), 7.14(dd, 2H), 7.19(d, 1H), 7.35(dd, 2H), 7.46(d, 1H) , 9.78 (s, 1H).
[0542] The following compounds were prepared analogously:
[0543] 1b 2-Cyclopentyl-N-(2-bromo-6-trifluoromethyl-4-morpholin-4-yl-phenyl)-acetamide.
[0544]...
Embodiment 2
[0562] 2a 2-Cyclopentyl-N-(2,6-dimethyl-4-thiomorpholin-4-yl-phenyl)-acetamide.
[0563] Bis(dibenzylideneacetone)palladium (37 mg) and (2'-dicyclohexylphophanyl-biphenyl-2-yl)-dimethyl-amine (38 mg) were desiccated in a dry atmosphere under argon atmosphere. Mix in degassed toluene (2 mL) for 5 minutes. To this mixture was added N-(4-bromo-2,6-dimethyl-phenyl)-2-cyclopentyl-acetamide (200 mg), potassium tert-butoxide (90 mg) and thiomorpholine (80 mg) and the reaction mixture was heated to 90 °C in a sealed 4 mL vial under argon atmosphere for 16 hours, cooled and filtered through silica (2 g). Water / brine (1:1, 4 mL, total) was added, and the mixture was extracted with ethyl acetate (3 x 2 mL). The combined organic phases were dried over magnesium sulfate and concentrated in vacuo. The crude product was purified by preparative LC-MS to provide 5.6 mg (3% yield) of the title compound. LC-MS-TOF (m / z) 333 (MH + ); R =2.03, (UV, ELSD) 98%, 100%.
[0564] The following co...
Embodiment 3
[0582] 3a 2-Bicyclo[2.2.1]hept-2-yl-N-(2,6-dimethyl-4-morpholin-4-yl-phenyl)-acetamide.
[0583] Bicyclo[2.2.1]hept-2-yl-acetic acid (0.41 g) was dissolved in a 1:1 mixture of thionyl chloride and 1,2-dichloroethane (10 mL, total) under argon atmosphere Heat to 50°C for 2 hours. The solvent was removed in vacuo, the resulting acid chloride was redissolved in acetonitrile (5 mL), and 2,6-dimethyl-4-morpholin-4-yl-aniline (0.50 g) was added. In a sealed microwaved vial, the reaction mixture was heated to 150°C for 10 minutes. Water (25 mL) was added and the product was extracted with ethyl acetate (3 x 25 mL). The combined organic phases were washed with brine (50 mL), dried over magnesium sulfate and concentrated in vacuo. The crude product was purified by flash chromatography to provide 0.074 g (9%) of the title compound as an off-white solid. LC-MS-TOF (m / z) 343 (MH + ); R =2.21, (UV, ELSD) 98%, 100%. 1 H NMR (500MHz, DMSO-d 6 ): 1.14(m, 4H), 1.41(m, 4H), 1.90(m, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com