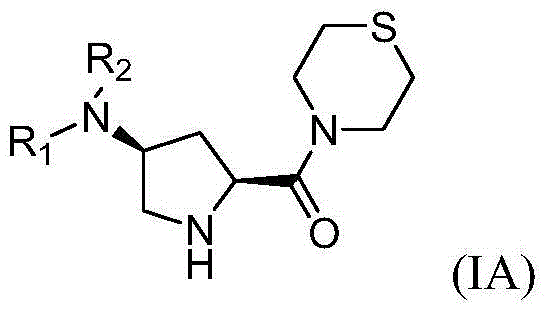
4-substituted pyrrolidine formyl thiomorpholine DPP-IV (Dipeptidyl Peptidase IV) inhibitor
A technology of alkyl and heterocycloalkyl, which can be used in metabolic diseases, medical preparations containing active ingredients, organic active ingredients, etc., and can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0120] Synthetic route of Scheme1 intermediate 5a
[0121]
[0122] Preparation of the first step 4-{[(2S,4R)-N-tert-butoxycarbonyl-4-hydroxy-pyrrolidin-2-yl]-formyl}thiomorpholine 2a
[0123] (2S,4R)-N-tert-butoxycarbonyl-4-hydroxypyrrolidine-2-carboxylic acid 1a (5.08g, 22mmol), HOBt (2.70g, 20mmol), diisopropylethylamine (5.2mL , 30mmol) and thiomorpholine (2mL, 20mmol) were placed in a 100mL single-necked bottle, 50mL of acetonitrile was added, the system was cloudy, then EDCI (4.22g, 22mmol) was added, stirred overnight at room temperature, and the system was clarified. Acetonitrile was distilled off, the system was diluted with 300 mL of ethyl acetate, and washed twice with saturated brine containing 0.5 N sodium hydroxide. The ethyl acetate layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain an off-white solid, which was slurried with ether and filtered to obtain Intermediate 2a, 4.79 g of a white sol...
Embodiment 1
[0150]
[0151] Compound 14-{[(2S,4S)-4-(N-Methyl-N-ethylamino)-pyrrolidin-2-yl]-formyl}-thiomorpholine dihydrochloride
[0152]
[0153] The first step 4-{[(2S,4S)-N-tert-butoxycarbonyl-4-(N-methyl-N-ethylamino)-pyrrolidin-2-yl]-formyl}thiomorpholine Preparation of 1c
[0154] 4-{[(2S,4S)-4-Methylamino-N-tert-butoxycarbonyl-pyrrolidin-2-yl]-formyl}thiomorpholine 6a (100 mg, 0.304 mmol) was dissolved in 4 mL 1,2 -Dichloroethane, add 2 drops of acetic acid dropwise, and add sodium triacetoxyborohydride (193mg, 0.912mmol) and acetaldehyde (0.11mL, 0.608mmol). Stir at room temperature for 1 h, add saturated sodium bicarbonate solution and stir for 5 min, and extract with dichloromethane three times. Combine the organic phases, wash with brine, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. ) mixture is the eluent. Intermediate 1c was obtained, white solid 94mg, yield 87.0%.
[0155] The second step of 4-{[(2S,4S)-4-(N-methyl-N-ethylamino)-py...
Embodiment 2
[0158]
[0159] Compound 24-{[(2S,4S)-4-(N-(4-trifluoromethylphenyl)-N-methylamino)-pyrrolidin-2-yl]-formyl}-thiomorpholine Dihydrochloride
[0160]
[0161] The first step 4-{[(2S,4S)-N-tert-butoxycarbonyl-4-(N-(4-trifluoromethylphenyl)-N-ethylamino)-pyrrolidin-2-yl] Preparation of -formyl}thiomorpholine 2c
[0162] 4-{[(2S,4S)-4-Methylamino-N-tert-butoxycarbonyl-pyrrolidin-2-yl]-formyl}thiomorpholine 6a (42mg, 0.128mmol) and 4-bromotri Fluorotoluene (32mg, 0.140mmol) was dissolved in 1mL of anhydrous toluene, cesium carbonate (58mg, 0.179mmol) was added, Pd(AcO) 2 (0.1%) and (±)-BINAP (0.3%), reacted at 100°C for 22 hours under the protection of argon, and then cooled to room temperature, filtered out the insoluble matter in the reaction system, and concentrated under reduced pressure. The obtained crude product was separated by column chromatography, using a dichloromethane:methanol (V:V=100:1.2) mixture as the eluent, to obtain intermediate 2c, 55 mg of white soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com