Maleic acid trimebutine slow release tablet comprising quick release part and preparing method thereof
A technology of trimebutine maleate and sustained-release tablets, applied in the direction of ester active ingredients, sugar-coated pills, pill delivery, etc., can solve the problems of slow onset of action and low bioavailability, and achieve good absorption and definite curative effect , the effect of taking convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 trimebutine maleate bilayer tablet (1000)
[0037] prescription:
[0038] 1. Immediate release layer
[0039] Trimebutine Maleate 150g
[0040] Microcrystalline Cellulose 50g
[0041] Tartaric acid 10g
[0044] 5% povidone absolute ethanol solution appropriate amount
[0045] A total of 1000 capsules
[0046] 2. Sustained release layer:
[0047] Trimebutine Maleate 150g
[0048] Lactose 10g
[0049] Hypromellose HPMC (K4M) 100g
[0050] Tartaric acid 10g
[0053] 5% povidone absolute ethanol solution appropriate amount
[0054] A total of 1000 capsules
[0055] Preparation Process:
[0056] 1. Immediate-release granules: weigh trimebutine maleate, tartaric acid, and microcrystalline cellulose in the prescribed amount of the immediate-release layer, mix wel...
Embodiment 2
[0060] Embodiment 2 trimebutine maleate sustained release tablet
[0061] Prescription: (based on 1000 tablets)
[0062] 1. Immediate Release Granules
[0063] Trimebutine Maleate 150g
[0064] Lactose 20g
[0065] Tartaric acid 10g
[0066] 5% povidone absolute ethanol solution appropriate amount
[0067] 2. Sustained-release granules:
[0068] Trimebutine Maleate 150g
[0069] Lactose 70g
[0070] Hypromellose HPMC (K4M) 200g
[0071] Tartaric acid 10g
[0072] 5% povidone absolute ethanol solution appropriate amount
[0073] Preparation Process:
[0074] 1. Immediate-release granules: weigh trimebutine maleate, tartaric acid, and lactose in the prescription amount of the immediate-release layer, mix well, add povidone ethanol solution to make a soft material, pass through a 24-mesh sieve twice to obtain granules, and dry at 40°C 30 minutes.
[0075] 2. Sustained-release granules: take by weighing the prescription amount of the sustained-release layer, trimebutin...
Embodiment 3
[0078] The mensuration of embodiment 3 release rate
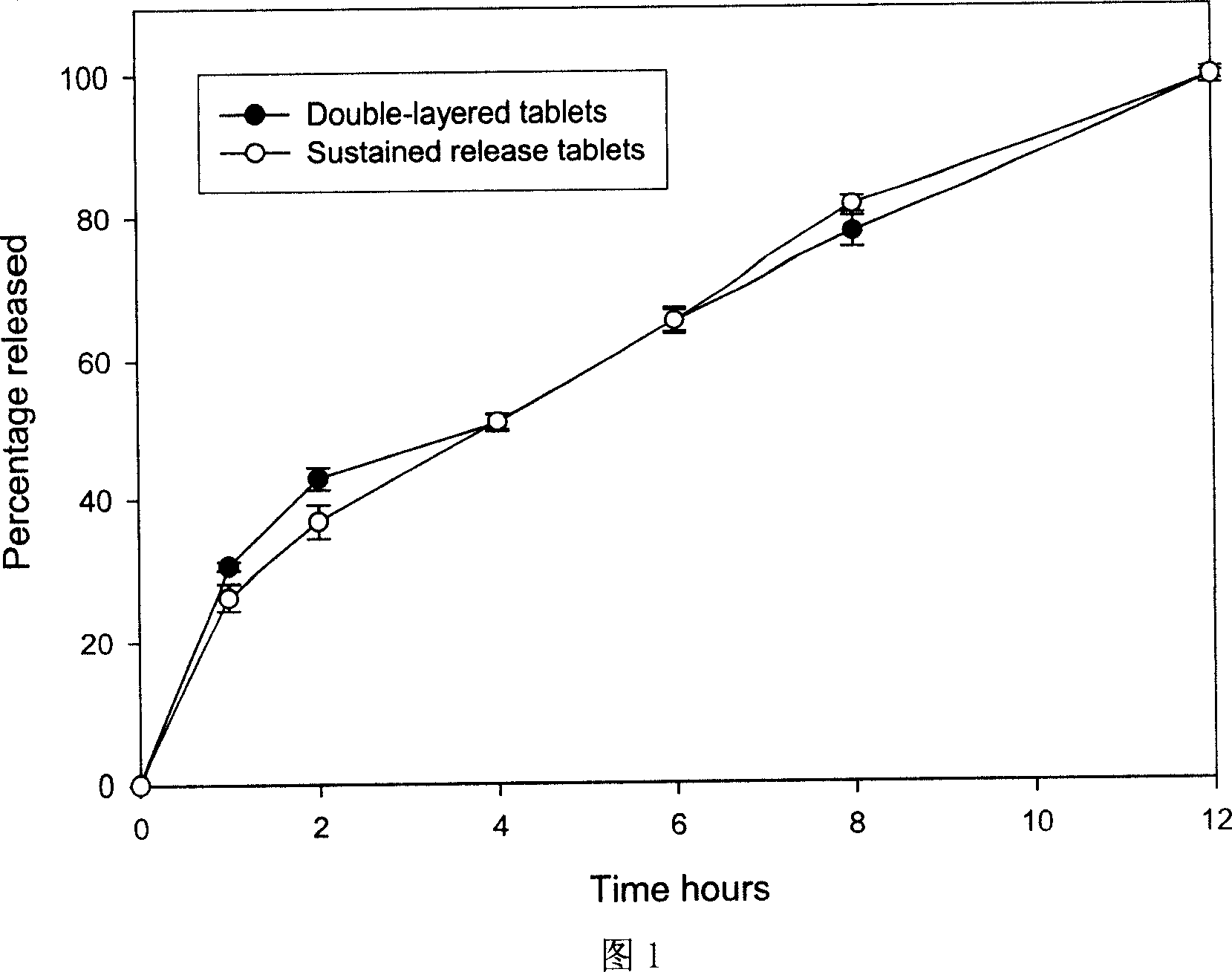
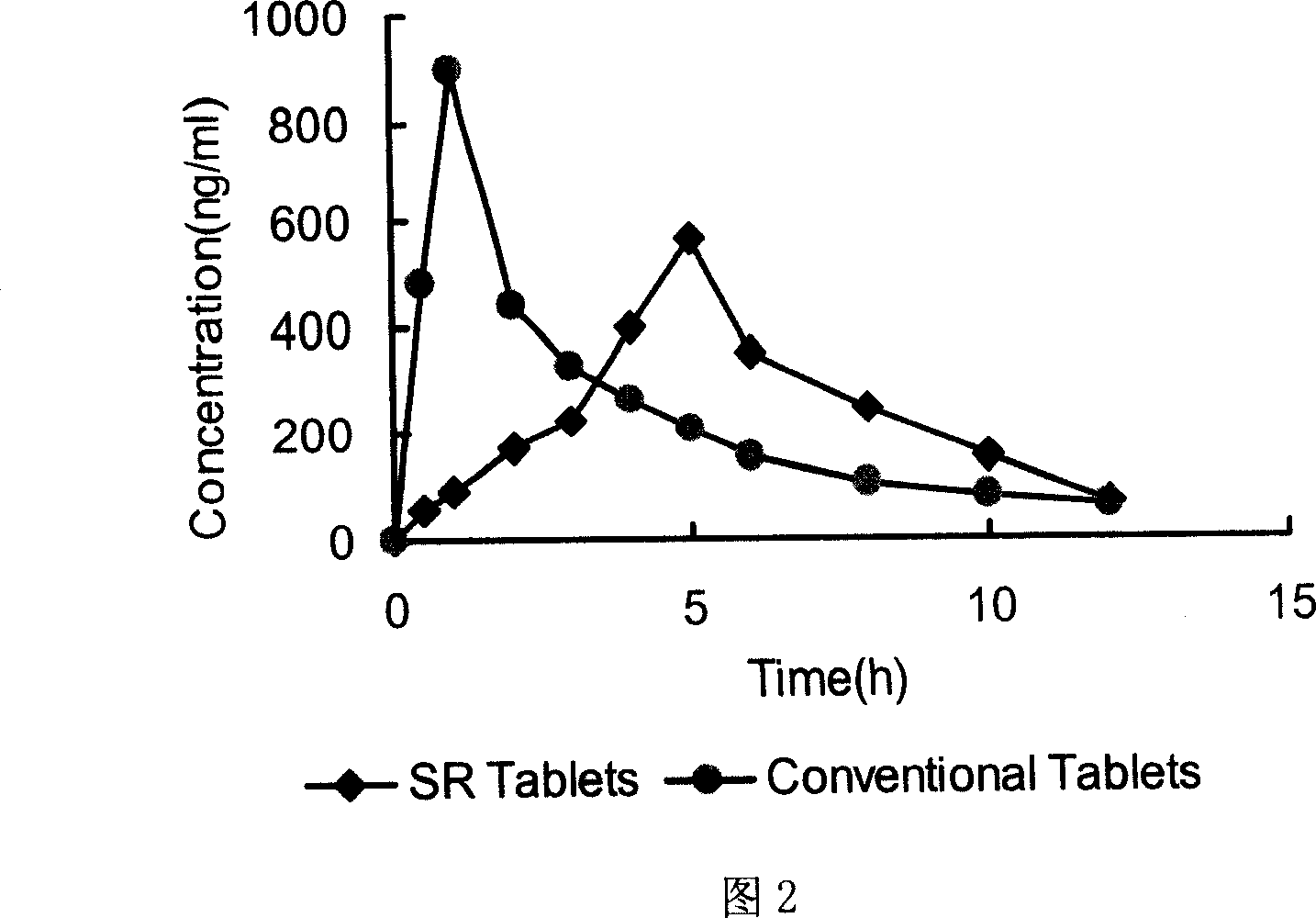
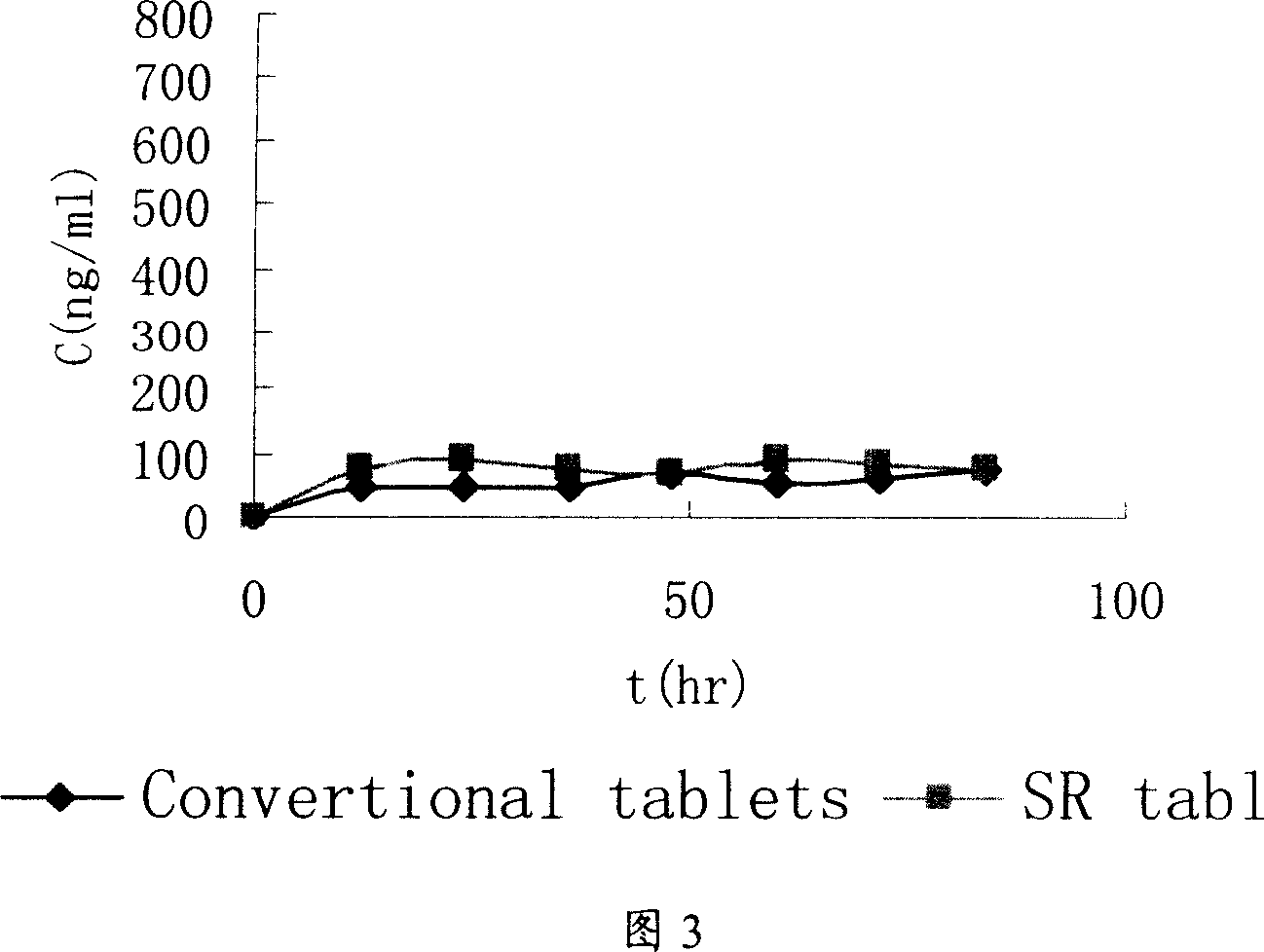
[0079] Get the trimebutine maleate slow-release tablet that obtains under embodiment 1, embodiment 2, according to release assay method (Chinese Pharmacopoeia version in 2005 two appendix XD first method), adopt dissolution assay (Chinese Pharmacopoeia 2005 edition two appendix XC first method) device, with 0.01mol / L hydrochloric acid solution 900ml as solvent, rotating speed is 100 revolutions per minute, operate according to law. Sampling 5ml at 1, 2, 3, 4, 6, 8, and 12 hours respectively, filtering, taking 2ml of the subsequent filtrate, putting it in a 10ml measuring bottle, adding 0.01mol / L hydrochloric acid solution to dilute to the mark as the test solution. In addition, accurately weigh an appropriate amount of trimebutine maleate reference substance, add 0.01mol / L hydrochloric acid solution to dissolve, quantitatively dilute to a solution with a concentration of about 20 μg / ml, and use it as the reference substance...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com