Synthesis of 2'-deoxy-l-nucleosides
A nucleoside and cyclic nucleoside technology, applied in the field of medicinal chemistry, can solve problems that cannot be used to treat disseminated tumor diseases, cell replication or cell metabolism interruption, and cannot be used to treat tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
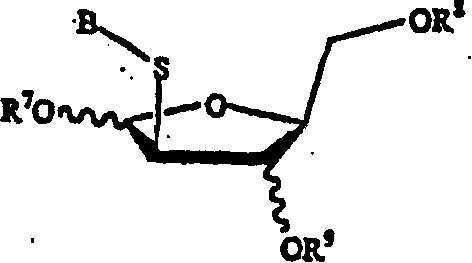
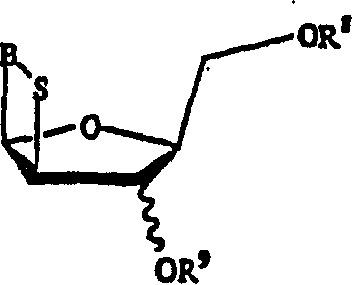
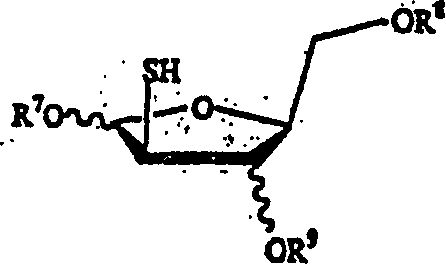
[0064] The present invention herein discloses the preparation method of the compound of formula (A).
[0065]
[0066] in
[0067] X and Y are independently hydrogen, OH, OR 1 , SH, SR 1 , NH 2 , NHR 1 or NR 1 R 2 ;
[0068] Z is hydrogen, halogen, CN or NH 2 ;
[0069] R is hydrogen, lower alkyl, aralkyl, halogen, NO 2 , NH 2 , NHR 3 , NR 3 R 4 , OH, OR 3 , SH, SR 3 , CN, CONH 2 , CSNH 2 , CO 2 H, CO 2 R 3 , CH 2 CO 2 H, CH 2 CO 2 R 3 , CH=CHR 3 , CH 2 CH=CHR 3 or C=CR 3 ;
[0070] R 1 , R 2 , R 3 and R 4 independently lower alkyl groups such as methyl, ethyl, propyl, butyl, and alkyl groups having 6 or fewer carbons, including cyclic, branched or straight chain, unsubstituted or substituted alkyl groups, wherein Alkyl groups may be substituted with one, two or more groups including but not limited to amino, carboxyl, hydroxyl and phenyl;
[0071] R 13 Is hydrogen, alkyl, acyl, phosphate (monophosphate, diphosphate, triphosphate or stabi...
Embodiment 1
[0619] 1-O-Acetyl-2,3,5-tri-O-benzoyl-β-L-ribofuranose (1,R 1 =Ac, R 2 =Bz)
[0620] 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose (1,R) was prepared from D-ribose as described by Recondo and Rinderknecht (loc. cit.) 1 =Ac, R 2 =Bz), the compound is prepared from L-ribose. A mixture of L-ribose (150 g, 1.0 mol) in methanol (2.5 L) containing 1% hydrochloric acid was stirred for 2 hours and then neutralized with pyridine (250 mL). The mixture was concentrated in vacuo and the residue was dissolved in pyridine (1 L). Benzoyl chloride (385 mL, 3.3 mol) was added dropwise to the solution while cooling to 0 °C. After overnight at room temperature, the mixture was concentrated in vacuo at 35-40 °C, and the residue was dissolved in ethyl acetate (1.5 L). Use cold water (2×0.5L), 1N H 2 SO 4 (3 × 0.5 mL), water (0.5 L) and saturated sodium bicarbonate (2 × 0.5 mL), the organic solution was washed successively, dried over magnesium sulfate, concentrated in vacuo to a slurry, ...
Embodiment 2
[0622] 2,3,5-Tri-O-benzoyl-D-ribofuranosyl bromide (2,X'=Br,R 2 =Bz)
[0623] Hydrogen bromide was bubbled into a solution of compound 1 (25.2 g, 0.05 mol) in ice-cold dichloromethane (150 mL) over 15 minutes. After 1 hour at 0°C and 15 minutes at room temperature, the solution was concentrated in vacuo. Continuous azeotropic distillation with toluene (25 mL×5) removed traces of hydrogen bromide. The syrupy residue (2) is immediately condensed with the appropriate purine or pyrimidine. of the slurry 1 The H-NMR spectrum includes a singlet δ6.5 (H-1, β-anomer) and a doublet 6.9 (H-1α-anomer, J 1,2 = 4.4 Hz). α / β is about 3:2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com