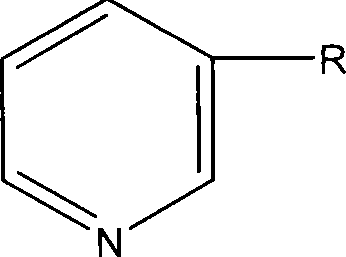
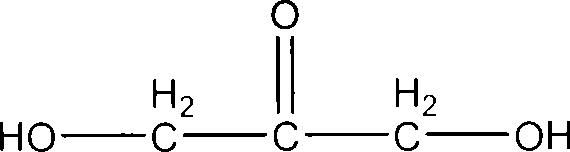
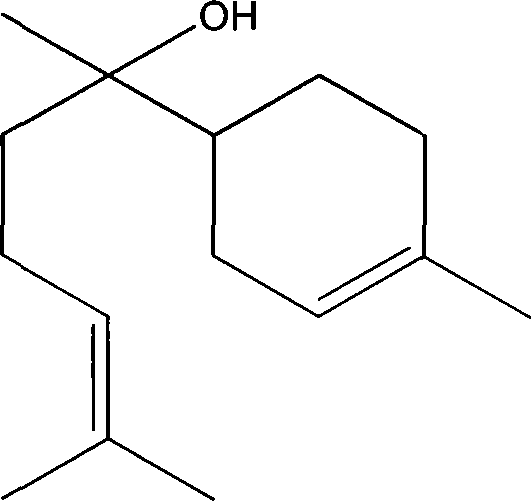
Formula including tetrapeptide and tripeptide mixture
A technology of formula and content, applied in the field of cosmetics and personal care products, can solve the problems of no improvement in this characteristic, no collagen stimulation effect of dipeptide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0302] Example 1: Anti-Wrinkle Night Cream
[0303] Product INCI Name %
[0304] Phase A
[0305] h 2 O 70.95
[0306] Ultrez 10 Carbomer 0.15
[0307] Phase B
[0308] Glycerine Glycerine 3.50
[0309] Phase C
[0310] Volpo S2 Steareth 2 0.40
[0311] Crodafos CES Cetearyl alcohol dicetyl
[0312] phosphate & ceteth
[0313] 10 phosphate 4.00
[0314] DC 345 Cyclohexasiloxane 2.00
[0315] Crodamol OSU Dioctyl succinate 7.00
[0316] Volpo S 10 Steareth 10 1.20
[0317] Nipastat Mixed parabens 0.30
[0318] D phase
[0319] Sorbate Sorbate Sorbate 0.10
[0320] Phase E
[0321] h 2 O 2.50
[0322] NaOH 38% Sodium hydroxide 0.30
[0323] F phase
[0324] Perfume Fragrance Fragrance 0.10
[0325] G phase
[0326] MATRIXYL® 3000 *) 3.00
[0327] The emulsion is prepared as follows: Phase A: Disperse Ultrez 10 in water and let it swell for 20 minutes, then add phase B; heat to 75°C B; heat phase C separately ...
example 2
[0330] Example 2: Decrease in the wrinkles around the eyes (in vivo)
[0331] Fifteen middle-aged female volunteers with wrinkles around the eyes participated in a study using the cream of Example 1. Wrinkles around the eyes were measured by questionnaires, a self-assessment scale and standard skin replication. Briefly, the method involves applying a polymer to the eye area which, when hardened, can be peeled away to reveal a "negative" contour of the skin surface. The contour instrument is then used to measure wrinkle depth, length, volume, etc. Then, according to the subject, the participants applied the cream product of Example 1 to the target area of one eye twice a day for 56 days; the other eye was coated with a control without peptide. The amount applied varies by subject. However, the amount should be the same for both eyes. Replicate assays were performed from day 0 to day 56. The results showed that in the eye area where the prescription of Example 1 was appli...
example 3
[0332] Example 3: Increase in synthesis of collagen (in vitro)
[0333]Human dermal fibroblasts were enriched with vitamin C (1.5 and 15 ppm) in the presence of the peptide N-palmitoyl-glycine-glutamine-proline-arginine (SEQ ID NO: 3). The peptides were cultured in two mediums for 72 hours, and there were two concentrations of peptides, namely 2.5ppm and 3.6ppm. After incubation, the medium was collected and the viability of the cells was assessed by the MTT assay. Collagen assays of each cell lysate were then performed by ELISA. The test was performed 3 times.
[0334] At the same time, a negative control should be tested under the same conditions but without the peptide.
[0335] The table of results in Table 2 below shows an increase in gelatin synthesis compared to the blank (no peptide) test:
[0336] N-Palmitoyl-Glycine-Glutamine-Proline-Arginine Concentration
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com