Method of purifying matter contaminated by heavy metal and apparatus therefor
A technology for purifying devices and pollutants, which is applied in the restoration of polluted soil and the removal of solid wastes, etc., and can solve the problems of miniaturization of difficult devices, inability to remove heavy metals, and reduction of sludge.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] [Example 1] Electrodeposition removal test of lead-contaminated soil
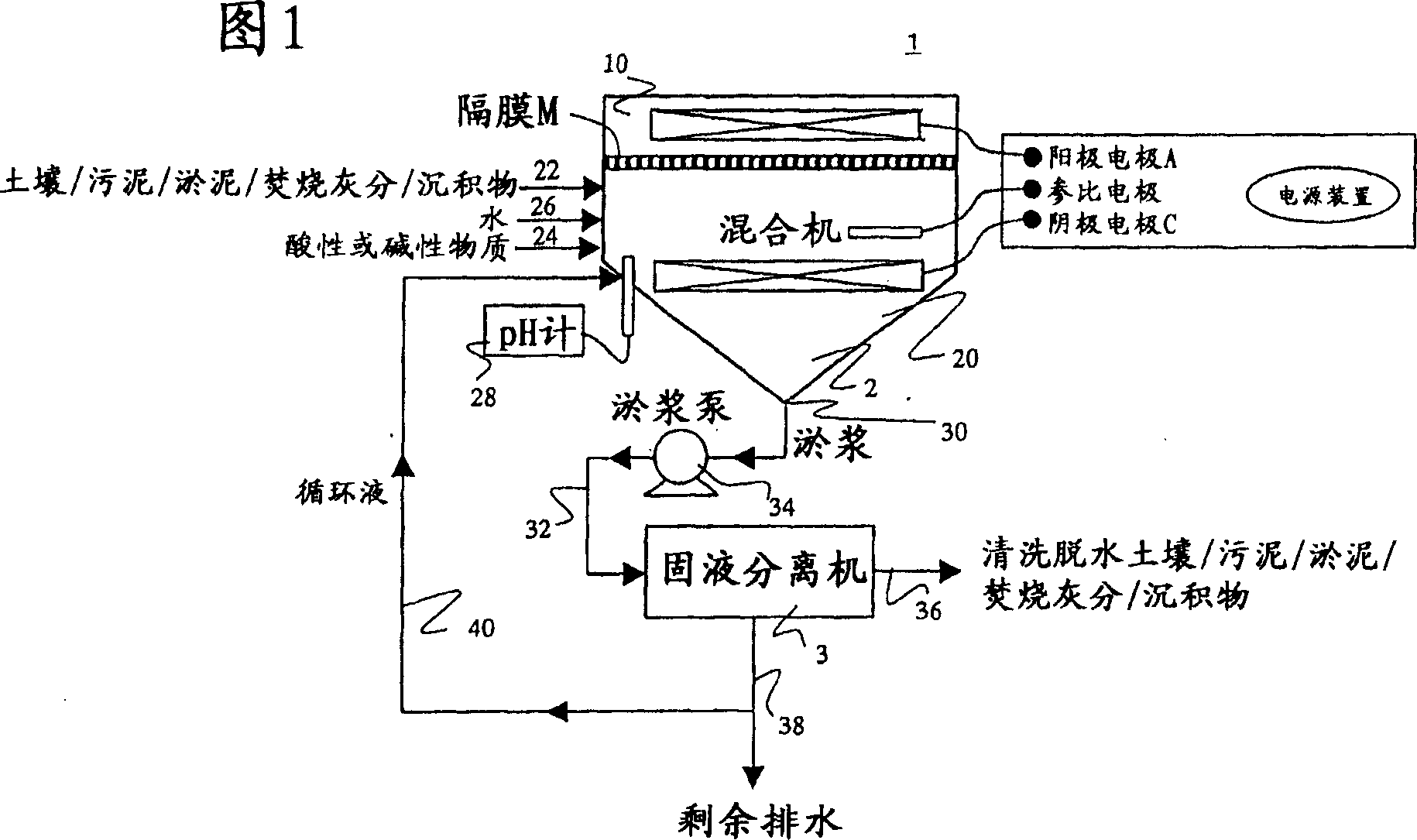
[0203] As shown in Figure 1, a cation exchange membrane (NEOSEPTA CMB manufactured by Tokuyama Co., Ltd.) was set in the central part of a reactor made of plexiglass with a volume of 2000 mL, and the reactor was divided into two regions: a cathode region and an anode region.
[0204] In the cathode area, add 100g of insoluble lead-contaminated soil (the lead concentration is 5000mg / kg dry soil; sampling location: A paint factory), 800mL of tap water, 50mL of 1:1 hydrochloric acid, using Teflon (registered trademark) The completed stirring blade was stirred at a speed of 500rpm to obtain the tested system.
[0205] Insert the cathode electrode of the copper metal mesh into the system under test, and connect it to the anode through a constant potential device (constant voltage power supply device).
[0206] In the anode region, 800 mL of tap water and 5 mL of 1:1 hydrochloric acid were added, and an a...
Embodiment 2
[0212] [Example 2] Electrode reduction cleaning test of mercury pollution deposit
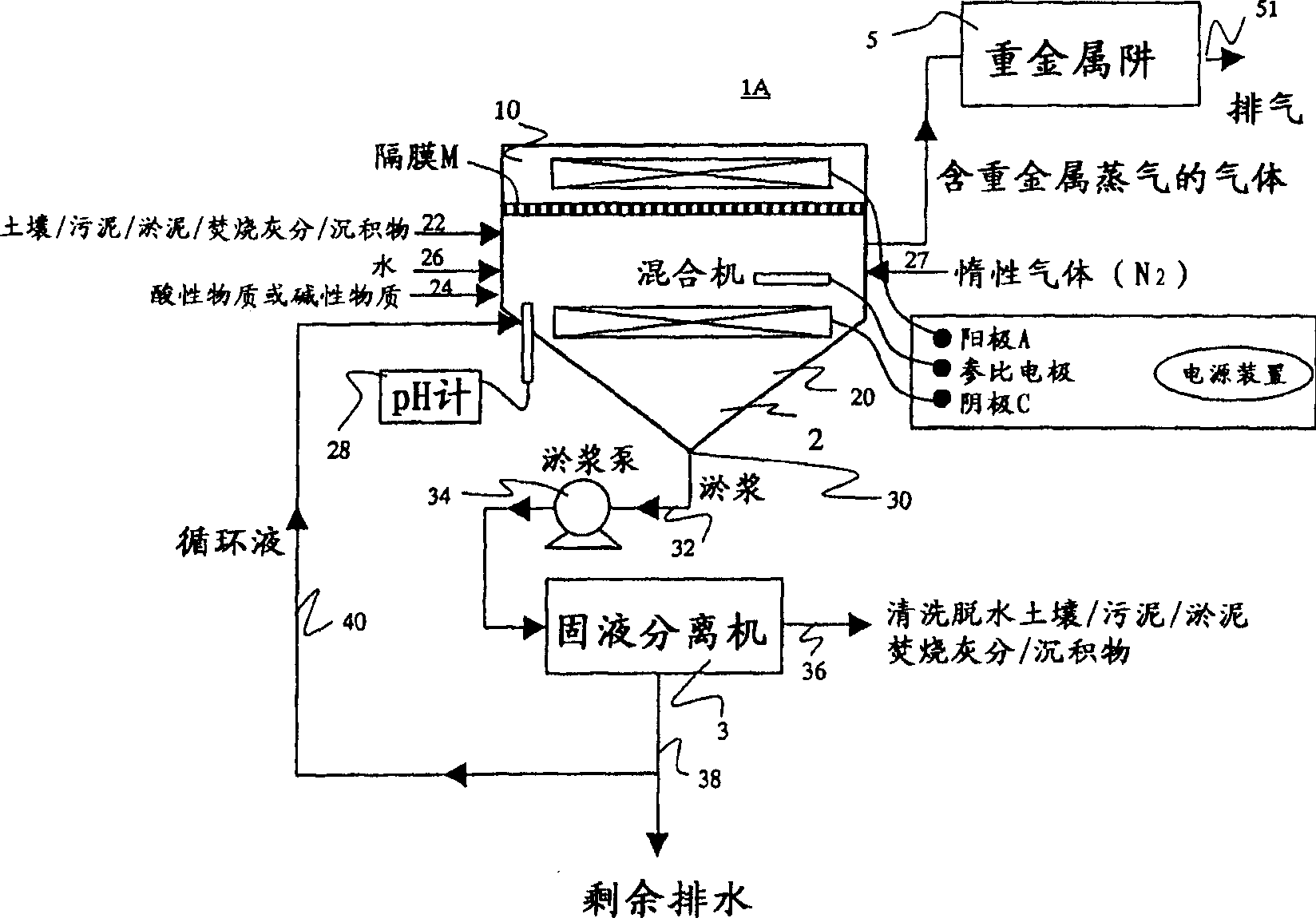
[0213] Such as figure 2 As shown, a cation exchange membrane (NEOSEPTA CMB manufactured by Tokuyama Co., Ltd.) is set in the central part of a reactor made of organic glass with a volume of 2000 mL, and the reactor is divided into two regions: a cathode region and an anode region.
[0214] In the cathode area, add 100g of insoluble lead-contaminated deposits (the total mercury concentration is 125mg / kg dry soil; sampling location: B pharmaceutical factory), 800mL of tap water, 50mL of 1:1 hydrochloric acid, use Teflon (registered trademark ) was stirred at a speed of 500rpm, a nitrogen diffuser pipe was inserted, and the cathode area was aerated with nitrogen at a speed of 10mL / min, which was used as the system under test.
[0215] Insert the cathode electrode of the titanium metal mesh into the system under test, and connect it to the anode electrode through a constant potential device. To ...
Embodiment 3
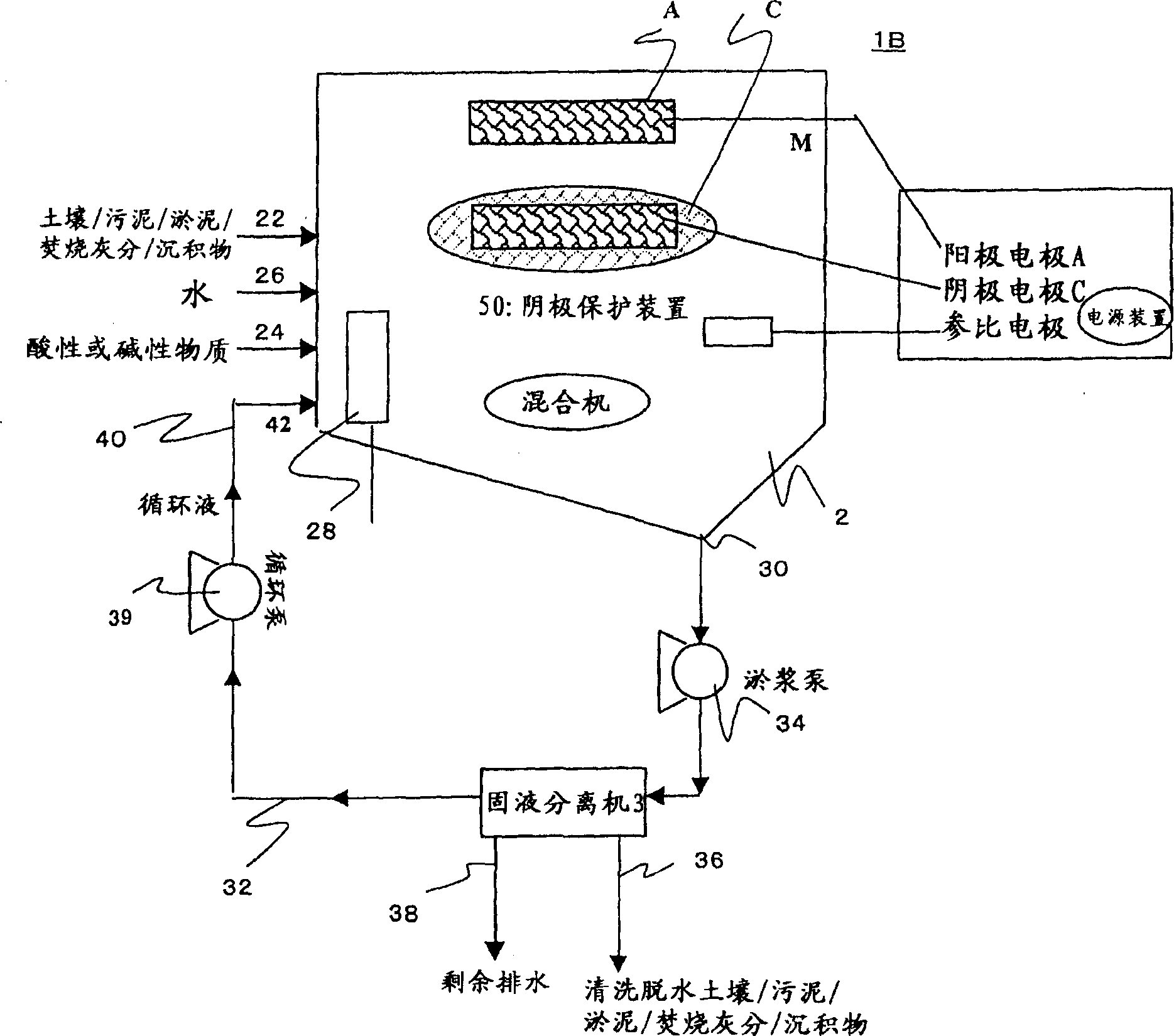
[0222] [Example 3] The influence of diaphragm on the electrolytic precipitation of lead-contaminated soil
[0223] As shown in Figure 1, a cation exchange membrane (NEOSEPTA CMB manufactured by Tokuyama Co., Ltd.) was set in the central part of a reactor made of plexiglass with a volume of 2000 mL, and the reactor was divided into two regions: a cathode region and an anode region. In the cathode area, add 100g of insoluble lead-contaminated soil (the lead concentration is 5000mg / kg dry soil; sampling location: A paint factory), 800mL of tap water, 50mL of 1:1 hydrochloric acid, using Teflon (registered trademark) The completed stirring blade was stirred at a speed of 500rpm to obtain the tested system. Insert the cathode electrode of the copper metal mesh into the test system, and connect it to the anode through a potentiostatic device (constant voltage power supply device). In the anode region, 800 mL of tap water and 5 mL of 1:1 hydrochloric acid were added, and a titanium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com