Coupling compound of tetracycline, preparation process and application thereof
A technology of tetracycline and conjugates, applied in chemical instruments and methods, biological testing, animal/human peptides, etc., can solve problems such as high price, short shelf life, and inability to meet detection needs, and achieve long-term compensation and saving The effect of testing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
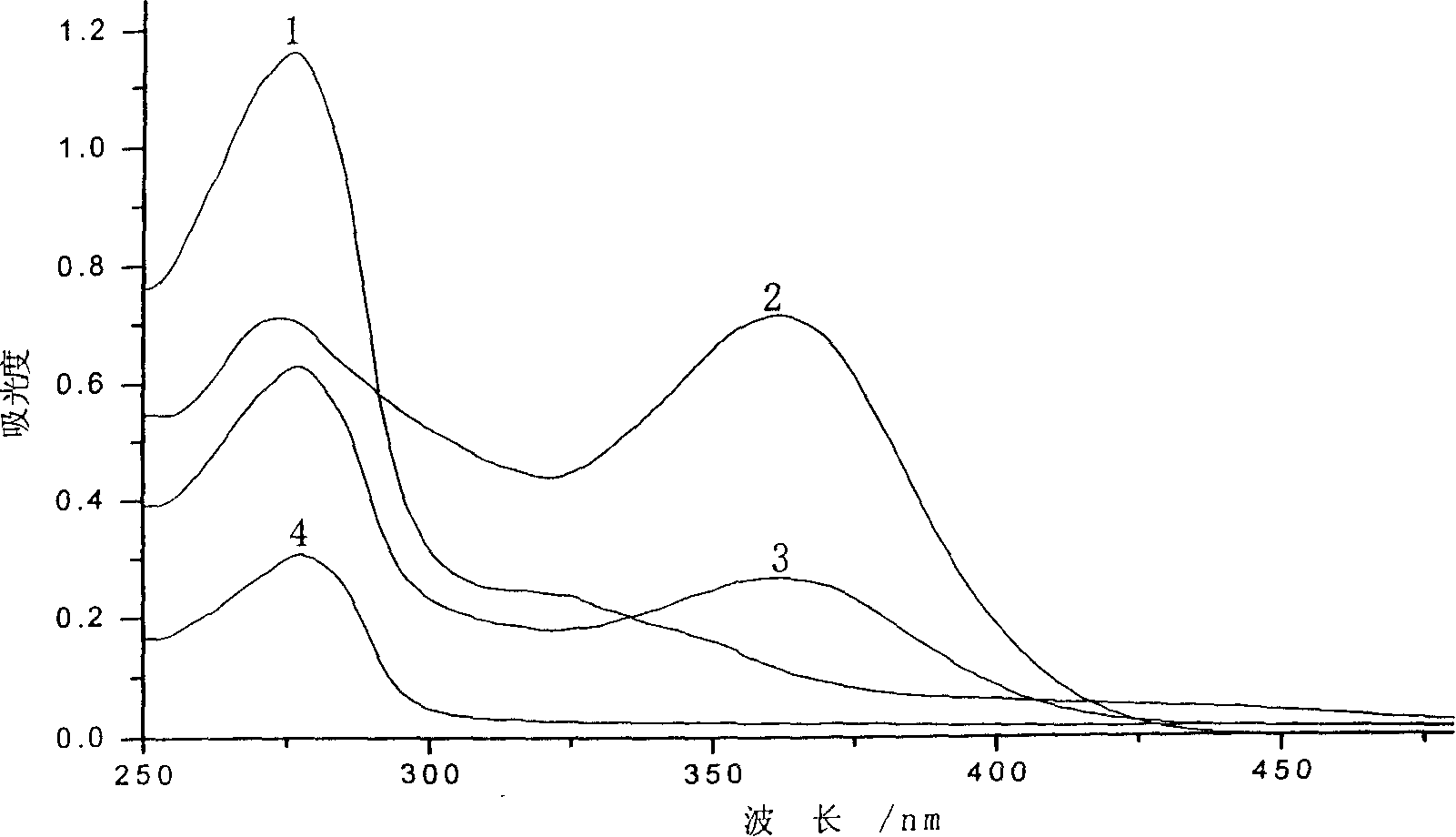
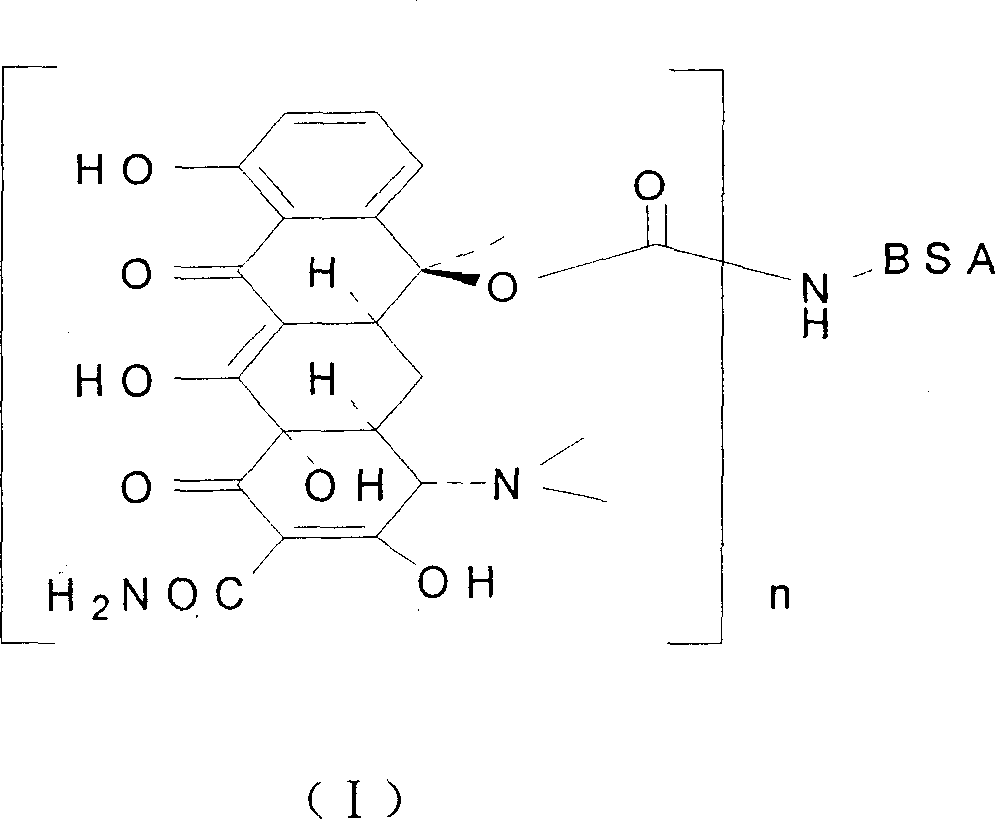
[0041] (1) Preparation of solution A: Dissolve 40 mg of tetracycline in 8 ml of anhydrous acetone, pass through nitrogen, then quickly add 29 mg of 1,1'-carbonyldiimidazole, and react in the dark at 37 ° C for 3 hours under nitrogen protection. Reaction generates the active intermediate of tetracycline and 1,1'-carbonyldiimidazole (CDI), for subsequent use; meanwhile, take 200mg bovine serum albumin (molecular weight is 6.8Kda) for subsequent use;
[0042](2) Preparation of solution B: the acetone in the above-mentioned reacted solution A was spun to dryness, and 15ml of pH=8.5 was added, and the concentration was 0.5M borax buffer solution to dissolve the generated tetracycline and the activity of 1,1'-carbonyldiimidazole intermediate, and then add the above-mentioned bovine serum albumin, so that the final concentration of bovine serum albumin is 13mg / ml, under the protection of nitrogen, 30 ℃ dark reaction for 48 hours; Stir dialysate solution B at -4°C for 72 hours, then d...
Embodiment 2
[0045] (1) Preparation of solution A: Dissolve 40 mg of tetracycline in 8 ml of anhydrous acetone, pass through nitrogen, then quickly add 16 mg of 1,1'-carbonyldiimidazole, and react in the dark at 37°C for 2 hours under nitrogen protection. Reaction generates the active intermediate of tetracycline and 1,1'-carbonyldiimidazole (CDI), for subsequent use; meanwhile, take 240mg bovine serum albumin (molecular weight is 6.7Kda) for subsequent use;
[0046] (2) Preparation of solution B: the acetone in the above-mentioned reacted solution A was spun to dryness, and 20ml of pH=9.0 was added, and the concentration was 0.5M borax buffer solution to dissolve the generated tetracycline and 1,1'-carbonyldiimidazole activity Intermediate, and then add the above-mentioned bovine serum albumin, so that the final concentration of bovine serum albumin is 12 mg / ml, under the protection of nitrogen, 25 ℃ dark reaction for 36 hours; Stir dialysate solution B at -4°C for 70 hours, then dialyze ...
Embodiment 3
[0049] (1) Preparation of solution A: Weigh 20 mg of tetracycline and dissolve it in 5 ml of anhydrous acetone, pass through nitrogen, then quickly add 10 mg of 1,1'-carbonyldiimidazole, and react in the dark at 37°C for 4 hours under the protection of nitrogen, Reaction generates the active intermediate of tetracycline and 1,1'-carbonyldiimidazole (CDI), for subsequent use; meanwhile, take 155mg bovine serum albumin (molecular weight is 6.9Kda) for subsequent use;
[0050] (2) Preparation of Solution B: Rotate the acetone in the A solution after the above reaction to dryness, add 15ml pH=8.0, and the concentration is 0.5M borax buffer solution to dissolve the generated tetracycline and 1,1'-carbonyldiimidazole activity intermediate, and then add the above-mentioned bovine serum albumin, so that the final concentration of bovine serum albumin is 10mg / ml, under the protection of nitrogen, 20 ℃ dark reaction for 40 hours; Stir dialysate solution B at -4°C for 75 hours, then dial...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com