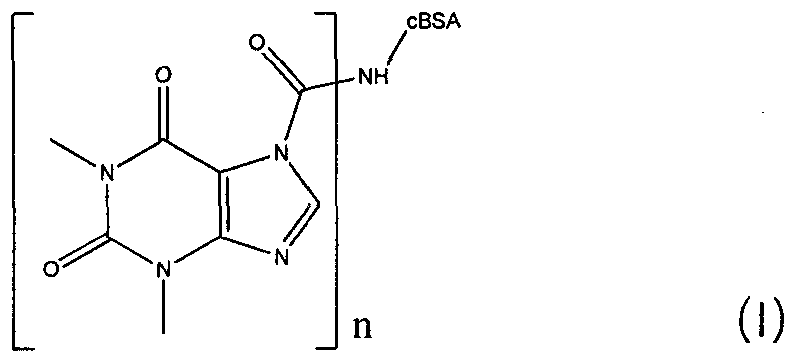
Theophylline conjugate and preparation method and application thereof
A conjugate, theophylline technology, applied in the field of clinical blood drug concentration monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0039] (1) Preparation of solution A: Weigh theophylline-7-acetic acid 7.4mg, CDI 15mg, DMAP 0.38mg and dissolve in 1mL DMF in turn, stir at room temperature overnight in the dark, and set aside;
[0040] (2) Preparation of cBSA: Under ice bath conditions, slowly drop ethylenediamine into PBS buffer solution with a pH of 7.4 and a concentration of 0.01M, stir at 0-4°C, and adjust the pH of the solution to 7.4 with concentrated HCl; weigh 1g BSA (15μmol) and 56mg EDC (300μmol) were added to the above solution of ethylenediamine, stirred and reacted at 20°C for 2 hours; the reaction solution of ethylenediamine and BSA was mixed with PBS buffer at 0-4°C (0.01M, pH 7.4) dialyzed for 70 hours, and then dialyzed with distilled water for 24 hours, changing the dialysate every 6 hours; centrifuging the dialyzed solution at 13,000 rpm for 15 minutes at 0-4°C, and taking the supernatant solution; freeze-dried supernatant to obtain cBSA as a white powder solid, which was used for future ...
Embodiment example 2
[0046](1) Preparation of solution A: Weigh 7.4 mg of theophylline-7-acetic acid, 30 mg of CDI, and 0.38 mg of DMAP, and dissolve them in 1 mL of DMF in turn, stir overnight at room temperature in the dark, and set aside;
[0047] (2) Preparation of cBSA: Under ice bath conditions, slowly drop ethylenediamine into PBS buffer solution with a pH of 7.4 and a concentration of 0.01M, stir at 0-4°C, and adjust the pH of the solution to 7.4 with concentrated HCl; weigh 1g BSA (15μmol) and 56mg EDC (300μmol) were added to the above solution of ethylenediamine, stirred and reacted at 25°C for 4 hours; the reaction solution of ethylenediamine and BSA was mixed with PBS buffer at 0-4°C (0.01M, pH 7.4) dialyzed for 72 hours, then dialyzed with distilled water for 30 hours, changing the dialysate every 6 hours; centrifuge the dialyzed solution at 13,000 rpm for 15 minutes at 0-4°C, and take the supernatant solution; freeze-dried supernatant to obtain cBSA as a white powder solid, which was...
Embodiment example 3
[0052] (1) Preparation of solution A: Weigh 7.4 mg of theophylline-7-acetic acid, 30 mg of CDI, and 0.76 mg of DMAP, and dissolve them in 1 mL of DMF in turn, stir overnight at room temperature in the dark, and set aside;
[0053] (2) Preparation of cBSA: Under ice bath conditions, slowly drop ethylenediamine into PBS buffer solution with a pH of 7.4 and a concentration of 0.01M, stir at 0-4°C, and adjust the pH of the solution to 7.4 with concentrated HCl; weigh 1g BSA (15μmol) and 56mg EDC (300μmol) were added to the above solution of ethylenediamine, stirred and reacted at 25°C for 4 hours; the reaction solution of ethylenediamine and BSA was mixed with PBS buffer at 0-4°C (0.01M, pH 7.4) dialyzed for 80 hours, then dialyzed with distilled water for 20 hours, changing the dialysate every 6 hours; centrifuge the dialyzed solution at 13,000 rpm for 15 minutes at 0-4°C, and take the supernatant solution; freeze-dried supernatant to obtain cBSA as a white powder solid, which wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com