Mumps virus ingredient vaccine for human, and its preparation method and uses
A mumps virus and vaccine technology, which is applied in the direction of antiviral agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the differences of different immunological effects, the immunological effect is not the best, and the incidence rate is reduced and other problems, to achieve the effect of good quality, high effectiveness, and reduced side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] ——Virus inactivation, lysis and purification
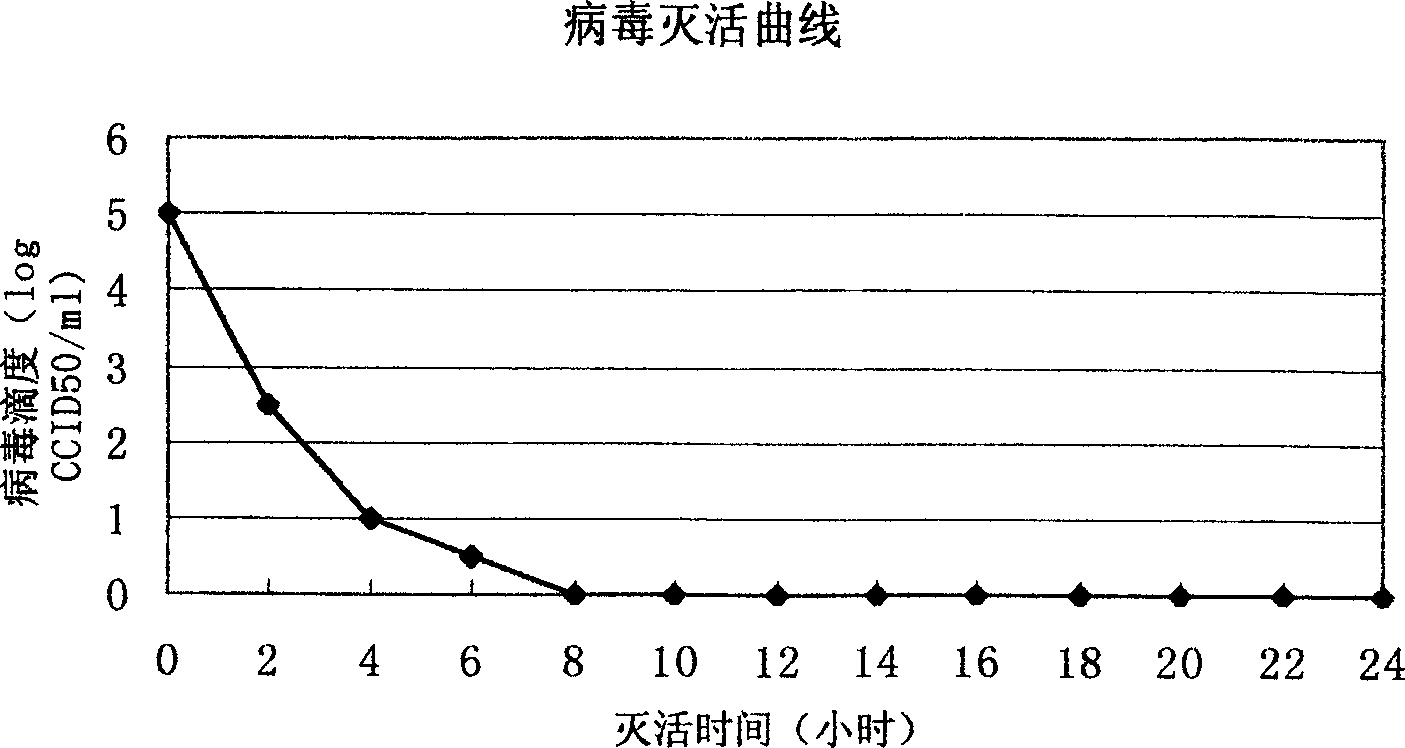
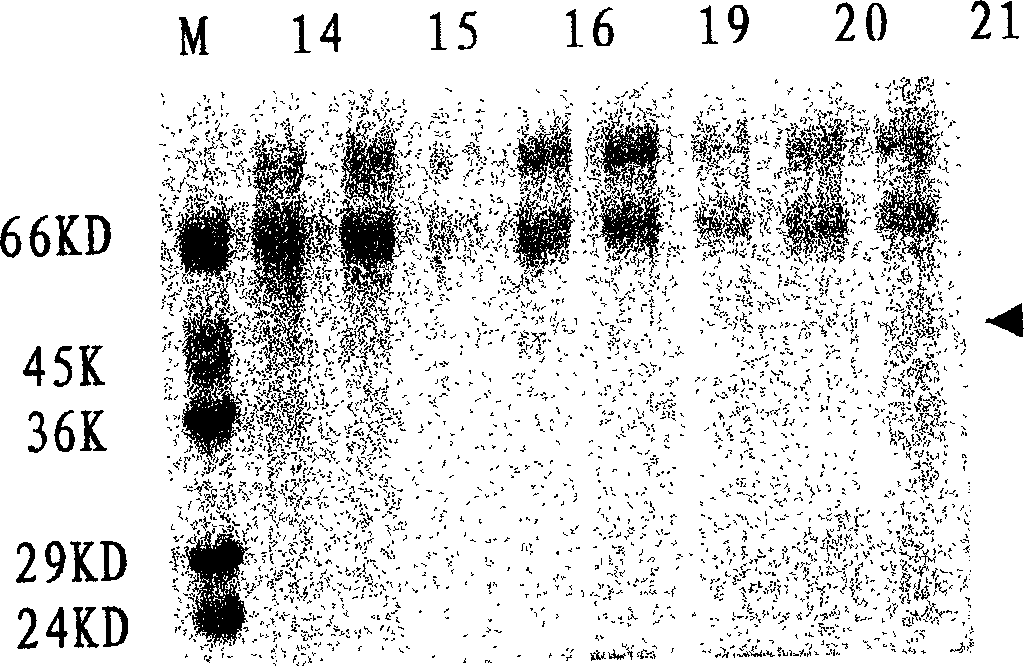
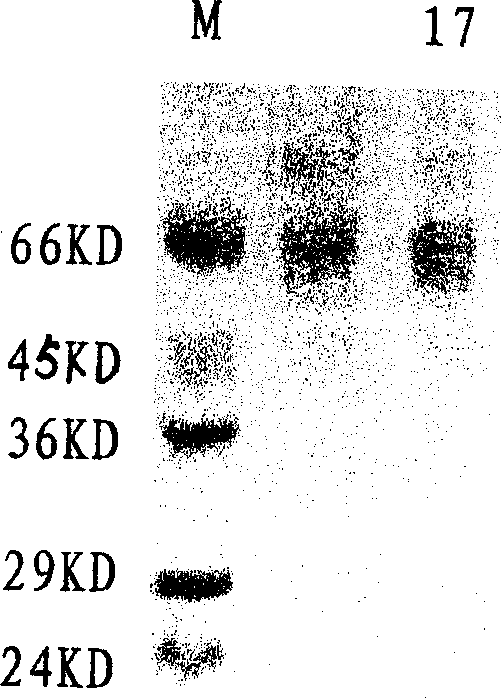
[0035] After the harvested virus liquid has been tested for titer and sterility, add formalin stock solution at 1:2000 (V / V), inactivate at 37°C for 7 days, take samples every 2 hours on the first day, and start on the second day Samples were taken daily to determine the virus inactivation curve, e.g. figure 1shown. After 7 days, the inactivated virus was concentrated by filtration and concentrated 100 times on the basis of the original volume, and the inactivated virus solution was added according to the proportion of TritonX-100 2%, SDS 1%, and allowed to react at room temperature for 2 hours. After the treatment, the inactivated virus liquid was filtered by the Sapharose4B column, and the separation results of the virus lysate were shown in Table 1, and the analysis results of the hemagglutination test were shown in Table 2. It was determined that the separated samples were taken, analyzed by PAGE-SDS (12%) electrophore...
Embodiment 2
[0039] ——Preparation of Experimental Mumps Virus Vaccine
[0040] According to the protein concentration of the semi-finished vaccine, dilute to 100μl / ml with physiological saline, and prepare 10.87mg / ml of Al(OH) at the same time 3 , the two are mixed in double (V / V) to make an experimental component vaccine, which is the developed mumps virus component vaccine of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com