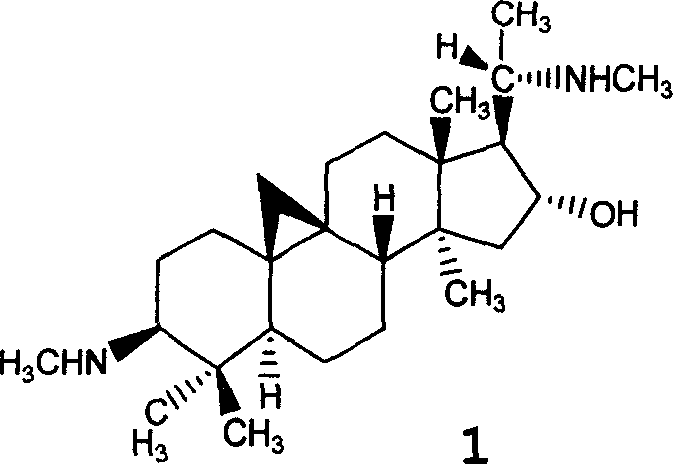
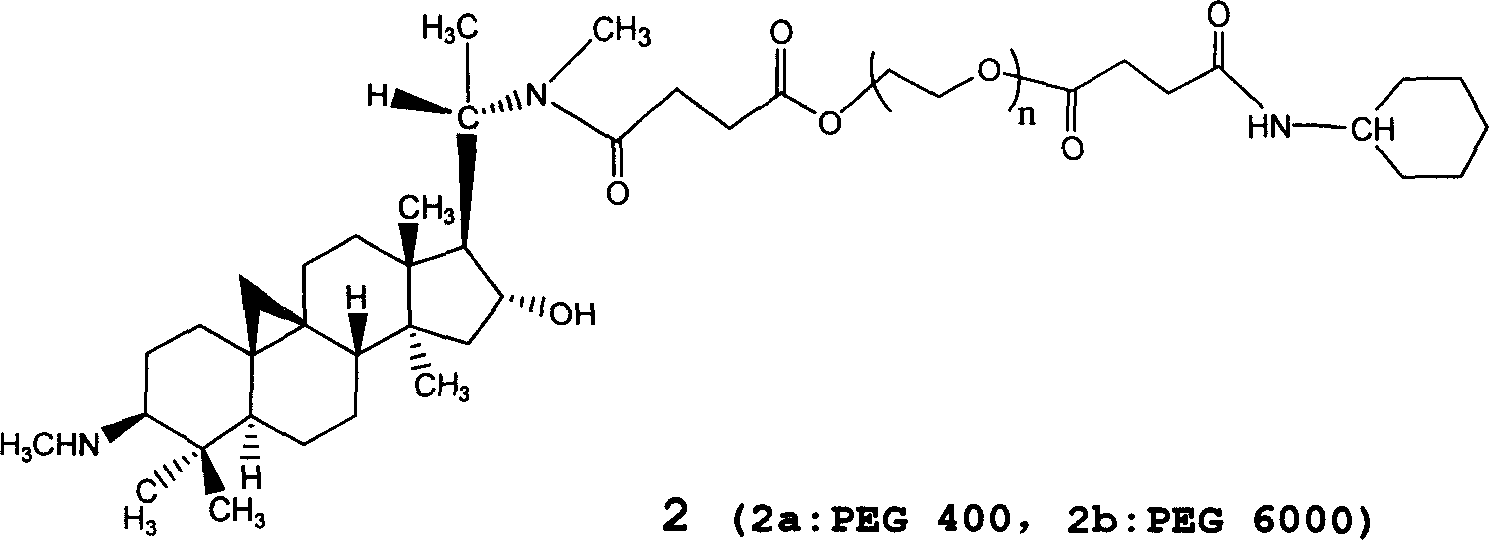
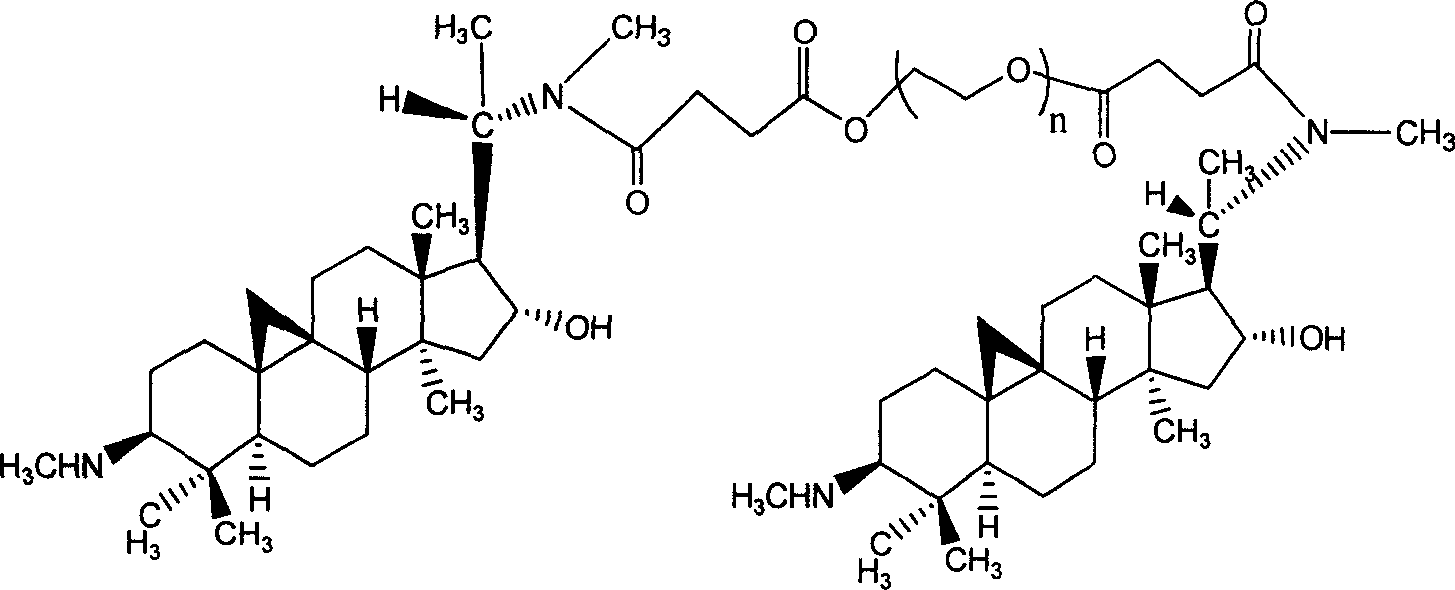
First-class new medicine cyclovirobuxine D series for treating cardiovascular and cerebrovascular disease and method for preparing the same
A technology for cardiovascular and cerebrovascular diseases, cyclovirboxicin, applied in the field of a new drug pegylated cyclovirboxicin D series for the treatment of cardiovascular and cerebrovascular diseases and its preparation field, which can solve the problem that the water solubility has not been well improved , poor water solubility, narrow safety range, etc., to achieve good blood compatibility and physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of polyethylene glycol-monoacid 5 (PEG-DA)
[0021] 60g (10mmol) PEG-6000 dissolved in 150mL anhydrous chloroform (CaH 2 Distilled off in the presence), 5g (50mmol) of succinic anhydride, 4mL of dry pyridine were added, and the reaction was refluxed under stirring for 48h. The reaction mixture was evaporated to dryness under reduced pressure, and the residue was saturated with NaHCO 3 Dissolve the aqueous solution, filter, cool the filtrate to 0~5℃, acidify with hydrochloric acid, extract three times with chloroform, wash the combined chloroform solution three times with water, anhydrous Na 2 SO 4 After drying and removing the desiccant, the filtrate is concentrated, and a large amount of dry ether is added to precipitate the product. The product was recrystallized three times with ether, and then weighed after drying. The product weighed 57 g and the yield was 90%.
[0022] According to this method, PEG-400-diacid, PEG-1000-diacid, PEG-2000-diacid, PEG-4000-diaci...
Embodiment 2
[0033]Take 150g of PEG-6000-diacid-CVB-D, add 20000ml of refined water for injection, dissolve, filter, prepare 2ml / bottle, sterilize, and get 10,000 water injections. Each bottle contains 15mg of PEG-6000-Diacid-CVB-D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com