Process for separating and determining pregabalin/Lyrica chiral isomer
A chiral isomer, pregabalin technology, applied in the field of analytical chemistry, can solve problems such as high cost and difficult separation, and achieve the effects of fast reaction speed, high analytical sensitivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Instruments and Conditions
[0032] U.S. Agilent 1100 high performance liquid chromatography system and workstation; automatic sampling; chromatographic column adopts octadecylsilane bonded silica gel column; ultraviolet detection wavelength: 340nm; mobile phase: triethylamine solution (take 5ml of triethylamine, add water 1000ml, adjust the pH value to 3.0 with phosphoric acid solution)—acetonitrile (55:45).
[0033] Experimental procedure
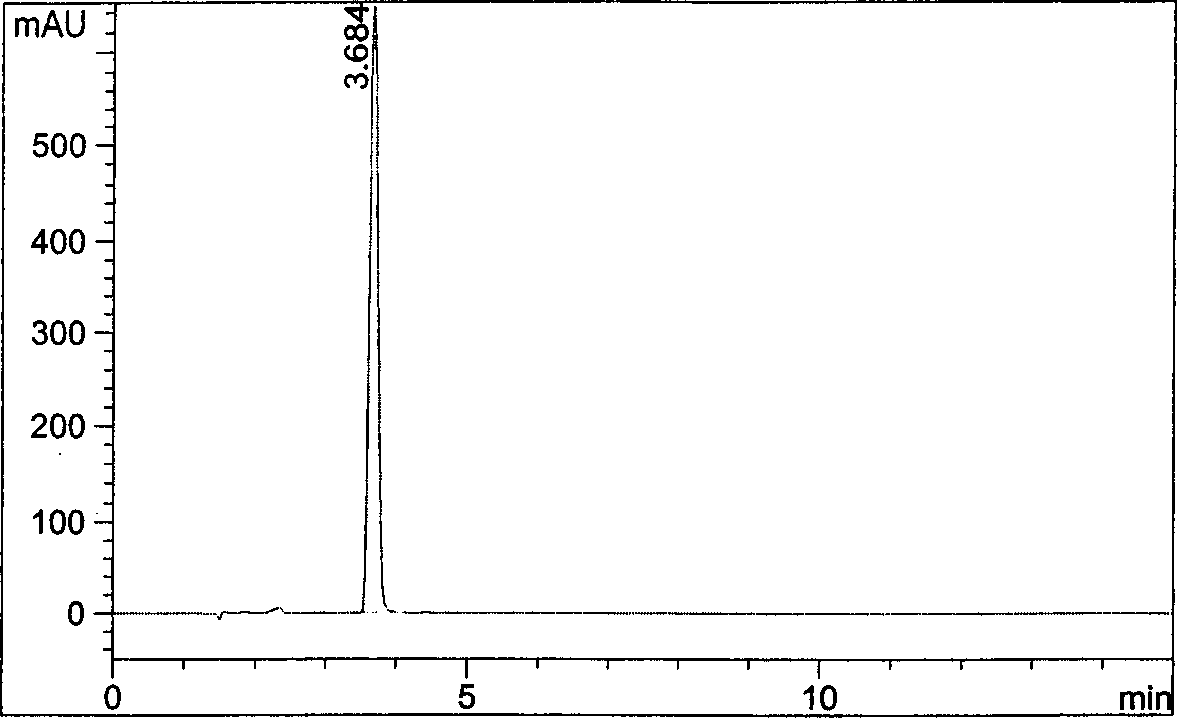
[0034] Take about 160 mg of the racemate of pregabalin into a 10 ml measuring bottle, take an appropriate amount of 1 mol / L hydrochloric acid solution, shake to dissolve, neutralize with 1 mol / L sodium hydroxide solution, and dilute to the mark with water. Shake well as a sample solution; take another 10.0 mg of Nα-(5-fluoro-2,4-dinitrophenyl)-L-alanamine amide, add 1 ml of acetone, shake well, and use it as a derivatization reagent solution. Take 50 μl of sample solution, 200 μl of derivatization reagent solution and 20 μl of 1m...
Embodiment 2
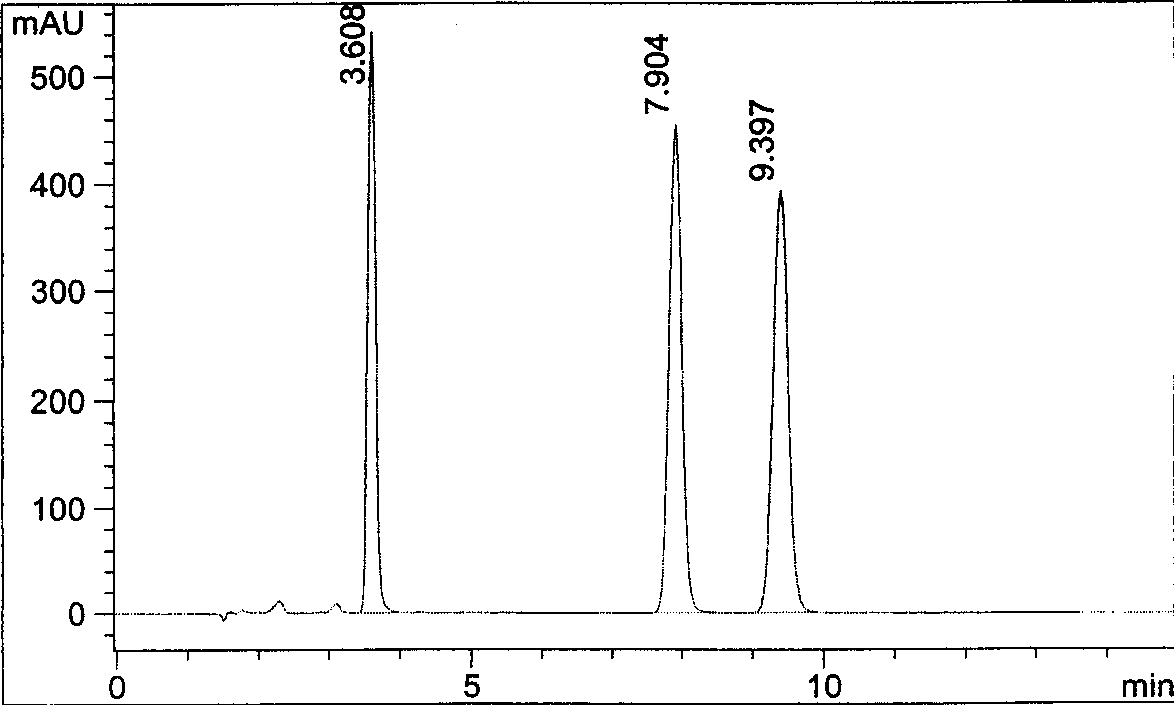
[0037]Get in about 80mg of pregabalin to 10ml measuring bottle, prepare need testing solution according to the experimental procedure of embodiment 1 below, and carry out high performance liquid chromatography analysis under the condition of embodiment 1, the results are shown in image 3 .
[0038] image 3 The chromatographic peak with a retention time of 3.666 minutes is an excess derivatization reagent peak; the chromatographic peaks with a retention time of 8.212 minutes and 9.9.8067 minutes are respectively pregabalin (S configuration) and its chiral isomer impurities (R configuration , 0.5%) chromatographic peak. image 3 It shows that pregabalin and its chiral isomers and excess derivatization reagent can achieve baseline separation, and can accurately determine the chiral isomer impurities contained in pregabalin.
Embodiment 3
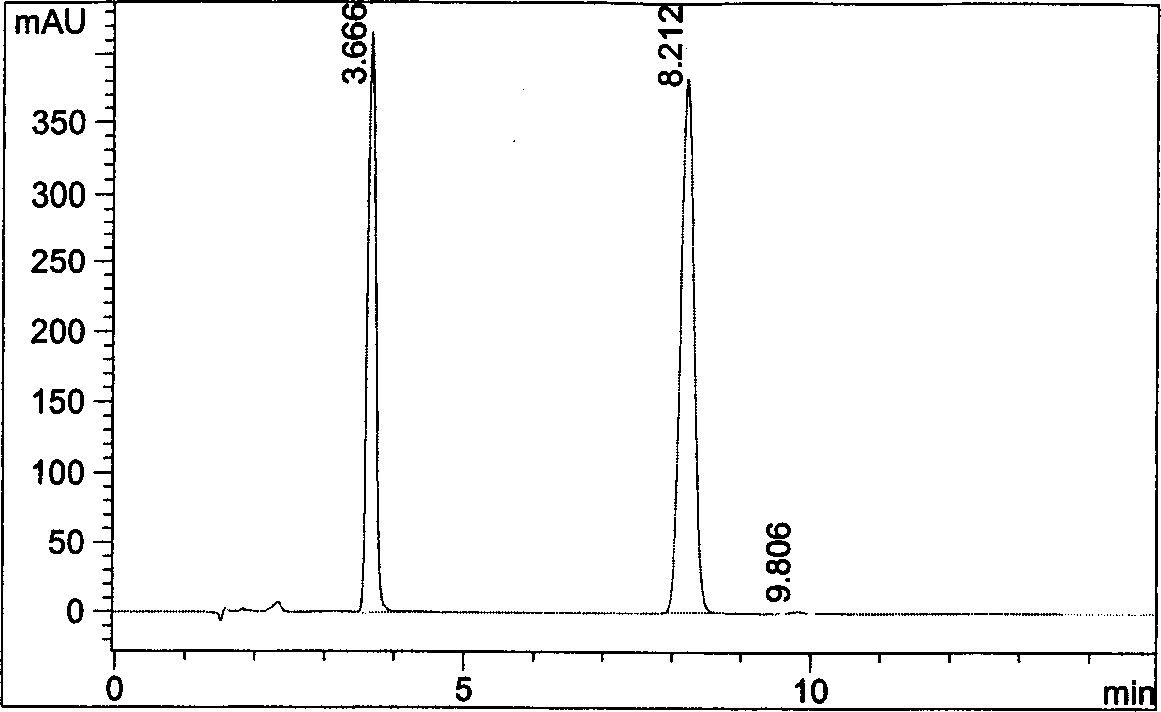
[0040] Get an appropriate amount of pregabalin capsule content (approximately equivalent to 80 mg of pregabalin) in a 10ml measuring bottle, prepare the test solution according to the experimental procedure of Example 1 below, and carry out HPLC analysis according to the conditions of Example 1 , see the result Figure 4 .
[0041] Figure 4 The chromatographic peak with a retention time of 3.633 minutes is the peak of excess derivatization reagent; the chromatographic peak with a retention time of 8.116 minutes is the chromatographic peak of pregabalin. Figure 4 It shows that pregabalin and excess derivatization reagent can achieve baseline separation, and if the sample contains pregabalin chiral isomer impurity, it can be accurately determined by this method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com