Genetic drug for targeted treatment of liver cancer and its preparation process
A technology for the treatment of liver cancer and gene medicine, which is applied in the field of medical medicine and gene medicine, and can solve problems such as unsatisfactory curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
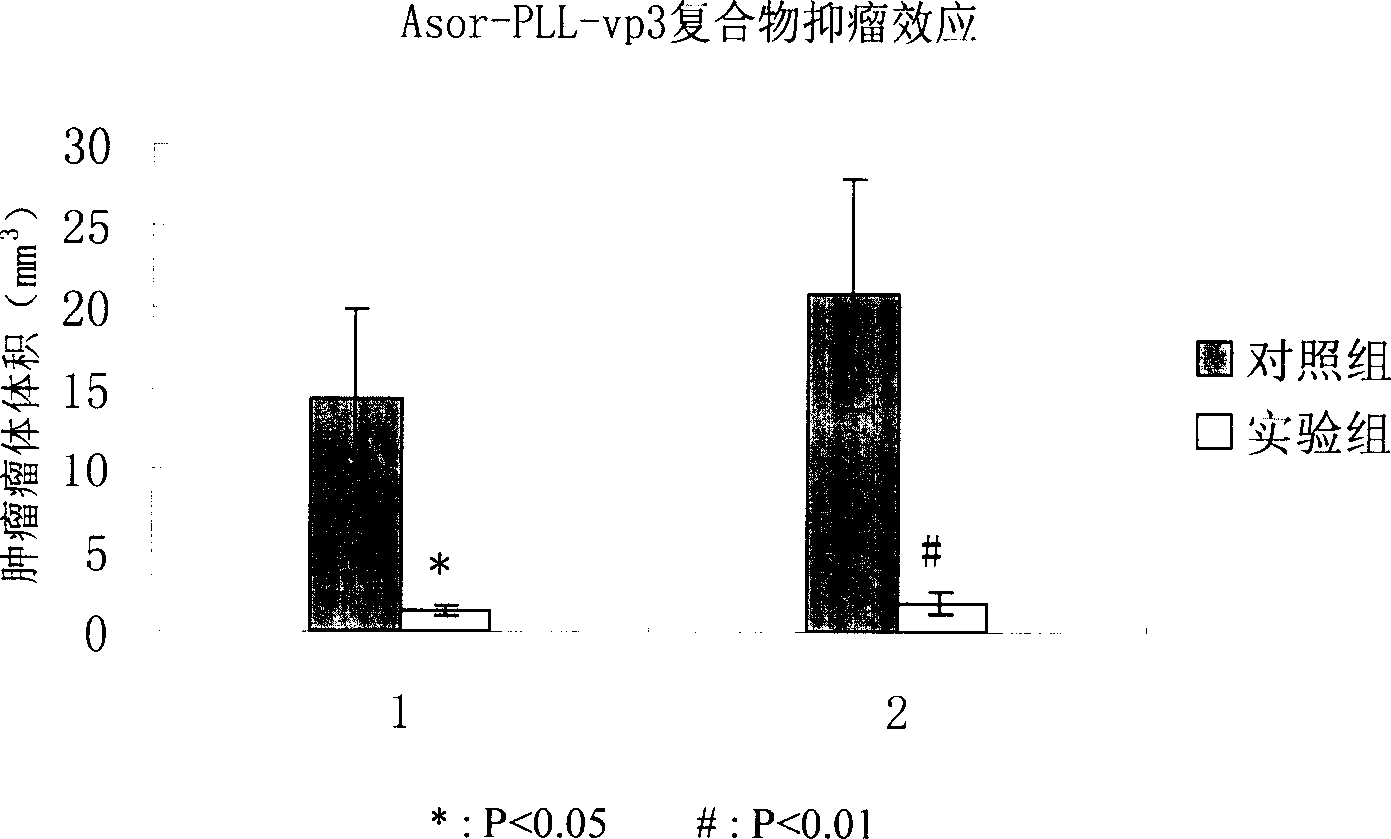
Image
Examples
Embodiment 1
[0019] (1) Preparation of Asor-PLL linker
[0020] Preparation of Asialomucin (Asor) from Human Plasma: Ion Exchange Chromatography and Ammonium Sulfate Fractionation Technique with DEAE-cellulose as Filler [See Whitehead P.H, Sammons H.G. et al Asimpletechnique for the isolation of orosomucoid from normal and pathological sera.Biochim.Bioiphy.Acta, 1966; 124:209-211], extract human serum mucin (Orosomucoid, OR) from human plasma, and then remove sialic acid in human serum mucin by acid hydrolysis , to produce asialo mucin (Asor).
[0021] Human serum mucinoid (Orosomucoid, OR)
[0022] ↓Acid hydrolysis to remove sialic acid
[0023] Asialomucin (Asor)
[0024] Link asialomucin (Asor) with poly-L-lysine (PLL) with protein cross-linking agent water-soluble carbodiimide (EDC) to prepare asialomucin (Asor)-poly-L-lysine Amino acid (PLL) linker (Asor-PLL), specifically, mix Asor, PLL, and EDC at a mass ratio of 1:1:0.5, dissolve in distilled water, adjust the pH to 7.2-7.4...
Embodiment 2
[0048] The asialyl mucin (Asor)-poly-L-lysine (PLL) linker and the pcDNA3 eukaryotic expression vector containing chicken anemia virus vp3 gene were dissolved in 0.9% NaCl solution respectively, and the two solutions were mixed by mass ratio ( Protein: nucleic acid) is 4: 1 fully mixed after, 37 ℃ places 16 hours, through filter sterilization, the prepared concentration is 200 μ g / ml (with the pcDNA3 eukaryotic expression vector containing chicken anemia virus vp3 gene in 0.9% NaCl solution Concentration calculated by mass) drug injection preparation of the present invention.
Embodiment 3
[0050] Asialomucin (Asor)-poly-L-lysine (PLL) linker and the pcDNA3 eukaryotic expression vector containing chicken anemia virus vp3 gene were dissolved in phosphate buffered saline (which consists of 0.8% NaCl, 0.02% KCl, 0.144% Na 2 HPO 4 , 0.024% KH 2 PO 4 ), the two solutions were fully mixed according to the mass ratio (protein:nucleic acid) of 3:1, placed at 37°C for 16 hours, and sterilized by filtration to obtain a concentration of 200 μg / ml (containing chicken anemia in phosphate buffer saline The concentration calculated by the mass of the pcDNA3 eukaryotic expression vector of the virus vp3 gene) the pharmaceutical injection preparation of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com