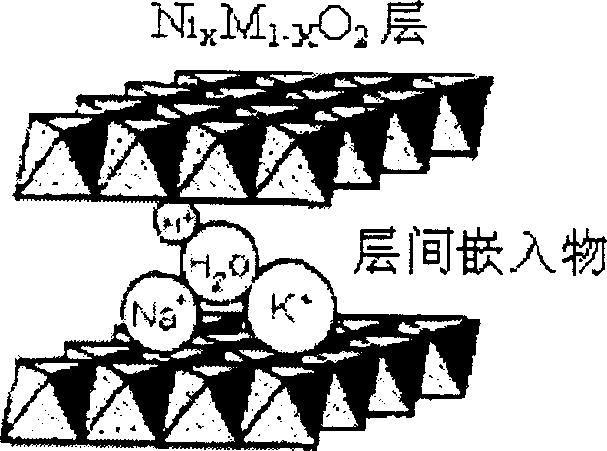
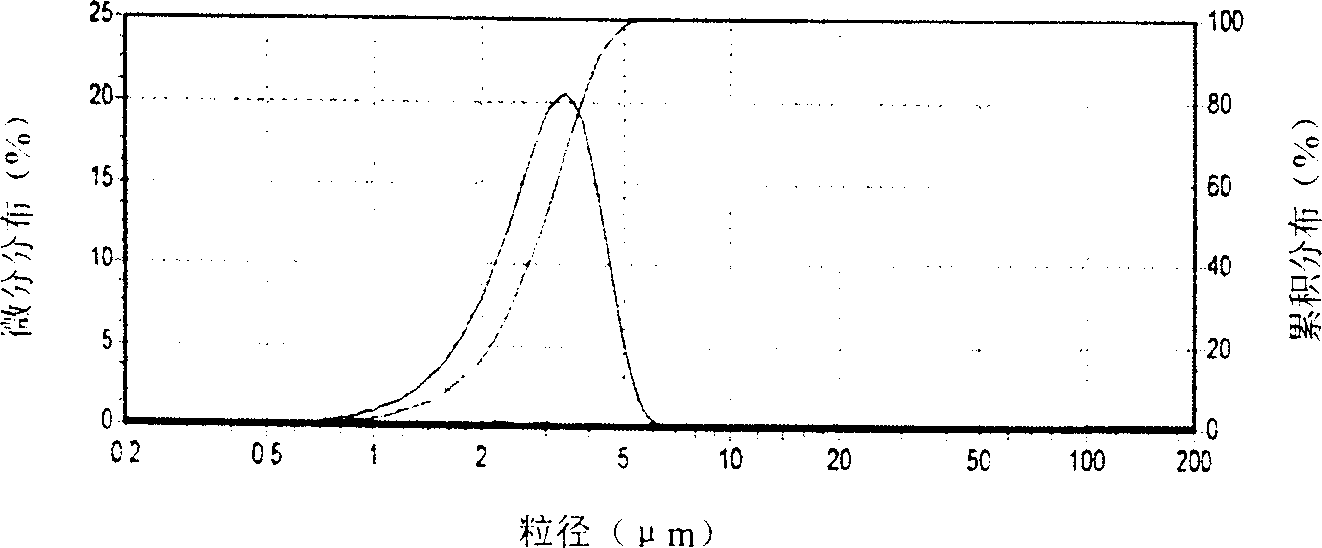
Spherical gamma nickel oxyhydroxide, preparation process and application thereof
A technology of γ-hydroxyl and nickel oxide, which is applied in nickel carbonyl, electrode manufacturing, electrical components, etc., can solve problems such as difficult self-discharge decomposition, poor battery storage performance, and low density of γ-NiOOH, achieving good crystallization state and density, Good stability and good storage performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare 2M nickel sulfate solution and 2M aluminum sulfate solution, mix 2M nickel sulfate solution and 2M aluminum sulfate solution to obtain solution A, the molar ratio of aluminum ion to nickel ion is 1:5; prepare 2M sodium hydroxide solution , and add 5% sodium carbonate to obtain solution B; prepare 10% ammonia solution to be solution C; solution A, B, and C are respectively placed in the high-level tank, and flow into the reactor, and the pH of the control reaction solution is 8.5 , the temperature of the reaction solution is 65°C, react for 18 hours, and stir the reaction solution; the solid-liquid mixture obtained by the reaction is subjected to conventional solid-liquid separation, the green solid is washed with distilled water, and dried at 60°C to obtain spherical α-nickel hydroxide Carry out electroless cobalt plating on the spherical α nickel hydroxide surface, and its cobalt content is 5% of spherical α nickel hydroxide; The spherical α nickel hydroxide obt...
Embodiment 2
[0036] Prepare 5M nickel nitrate solution and 5M manganese nitrate solution, mix 5M nickel nitrate solution and 5M manganese nitrate solution to obtain solution A, the molar ratio of manganese ions to nickel ions is 1:2; prepare 5M sodium hydroxide solution , and add 5% sodium carbonate to obtain solution B; prepare 20% ammonia solution to be solution C; solutions A, B, and C are respectively placed in the high-level tank, and flow into the reactor, and the pH of the control reaction solution is 9.5 , the temperature of the reaction solution is 60°C, react for 24 hours, and stir the reaction solution; the solid-liquid mixture obtained by the reaction is subjected to conventional solid-liquid separation, the green solid is washed with distilled water, and dried at 60°C to obtain spherical α-nickel hydroxide ; The obtained spherical α-nickel hydroxide was reacted with 2 times the amount of sodium persulfate in 2M potassium hydroxide solution, the temperature was 40°C, the time wa...
Embodiment 3
[0038]Prepare 1M nickel chloride solution and 1M cobalt chloride solution, mix 1M nickel chloride solution and 1M cobalt chloride solution to obtain solution A, the molar ratio of cobalt ion to nickel ion is 1:4; prepare 3M Sodium hydroxide solution and 5% sodium carbonate were added to obtain solution B; 15% ammonia solution was prepared as solution C; solutions A, B, and C were respectively placed in the high-level tank and flowed into the reactor to control the reaction solution. The pH of the solution is 9, the temperature of the reaction solution is 55°C, react for 36 hours, and stir the reaction solution; the solid-liquid mixture obtained by the reaction is subjected to conventional solid-liquid separation, the green solid is washed with distilled water, and dried at 80°C to obtain a spherical α-nickel hydroxide; the surface of spherical α-nickel hydroxide is electroless nickel-plated, and its nickel content is 6% of that of spherical α-nickel hydroxide; the spherical α-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com