Method for producing positive pole material-orthorhombic system LiMnO2 of lithium secondary battery
A lithium secondary battery, orthorhombic crystal system technology, applied in the field of battery material preparation, can solve problems such as difficult mass production, and achieve the effects of good crystallinity, abundant resources, and good electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Mn(CH 3 OO) 2 4H 2 O, MnO 2 , LiOH·H 2 O was added to 40mL distilled water, stirred at room temperature in air for 4 hours, then placed in a 40ml autoclave, kept at 170°C for 5 days, washed with dilute oxalic acid to neutral pH, and then deionized water Wash, and finally dewater and dry the precipitate to obtain orthorhombic LiMnO 2 powder.
[0019] In the hydrothermal synthesis of LiMnO 2 During the process, the pH of the test solution before pouring the reaction solution into the reactor was 14, showing strong alkalinity. After the constant temperature reaction at 170°C, the pH value of the solution was still 14 when it was taken out of the kettle. From this we can know that LiMnO 2 A strong alkaline environment is required in the hydrothermal synthesis process. Therefore, an excessive amount of lithium hydroxide (LiOH·H 2 O), to ensure that the reaction is carried out under a strongly alkaline environment.
Embodiment 2
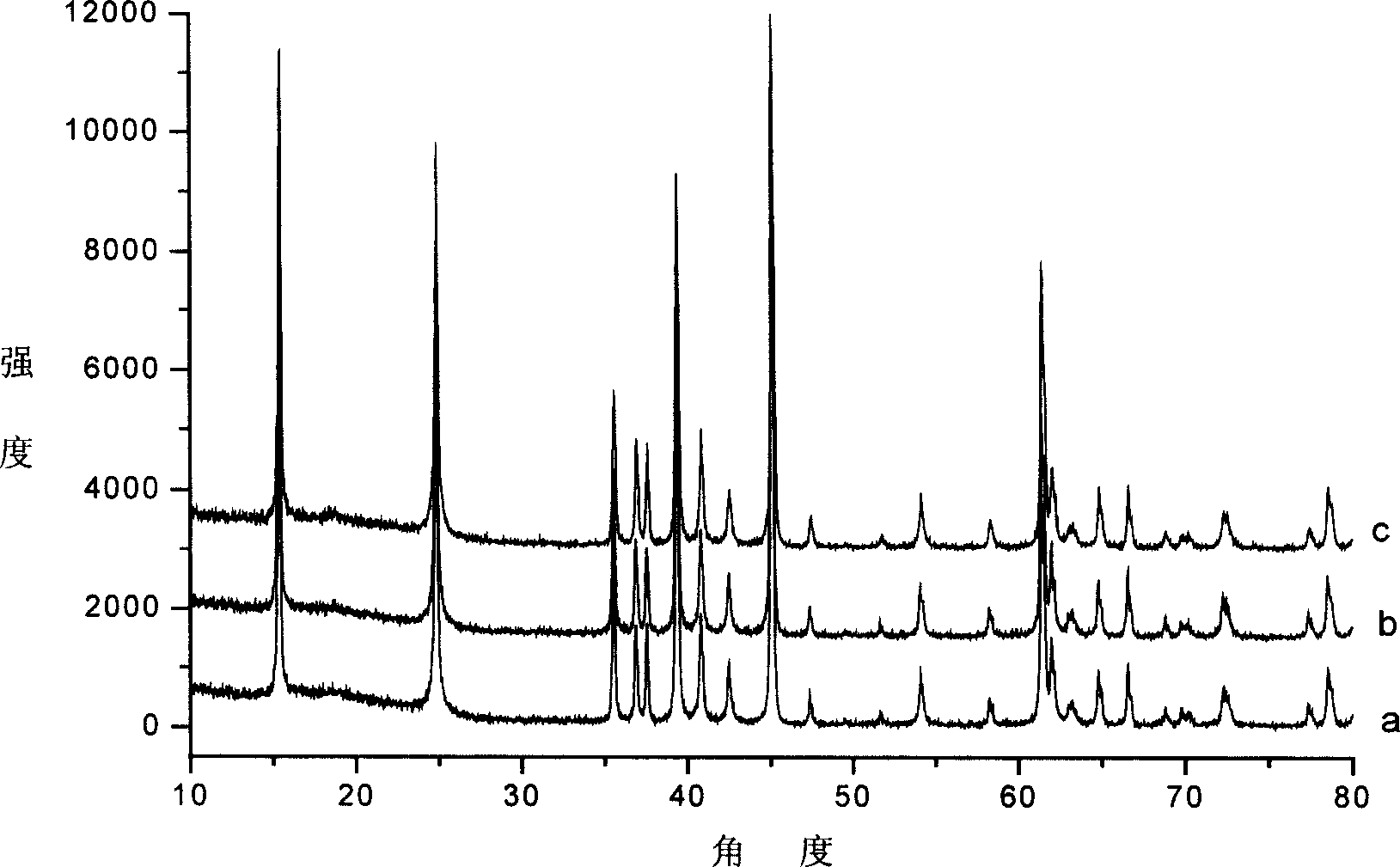
[0021] Select commercially available Mn(CH 3 OO) 2 4H 2 O, MnO with a molecular weight of 86.94 2 , LiOH·H with a molecular weight of 41.96 2 O was used as the starting material for the reaction. Mn(CH 3 OO) 2 4H 2 O, MnO 2 The consumption, hydrothermal synthesis temperature, reaction time and reaction process are identical with embodiment 1. Add raw material LiOH·H 2 The amount of O used is: 0.075mol; 0.095mol; 0.14mol, the resulting orthorhombic LiMnO 2 Performance, effect are basically the same as those in Example 1.
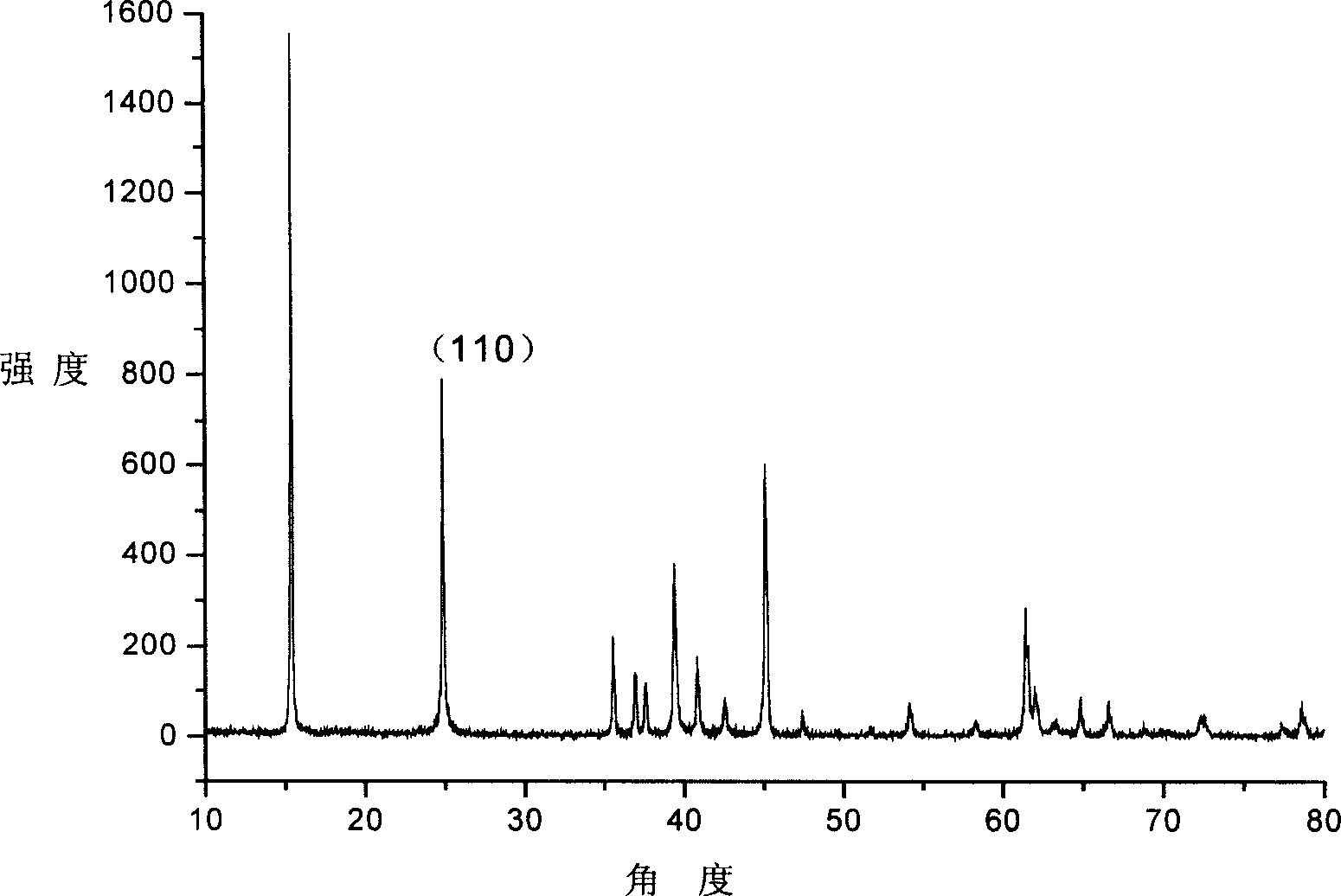
[0022] From the XRD spectrum of the sample, we can know that orthorhombic-LiMnO 2The half width of the peak increases slightly with the increase of LiOH concentration, but the maximum value is only 0.261° (as shown in Table 1). This shows that orthorhombic-LiMnO synthesized by hydrothermal method 2 The crystallinity is very good.
[0023] Table 1: Bandwidth values of four strong peaks in XRD spectrum
[0024]
Embodiment 3
[0026] The hydrothermal synthesis temperature, reaction time and reaction process are the same as in Example 1. In order to study the influence of different reaction raw materials on the reaction products, we selected manganese chloride (MnCl) with a molecular weight of 197.91 2 4H 2 O), MnO with a molecular weight of 86.94 2 , LiOH·H with a molecular weight of 41.96 2 O as the starting material for the reaction. The molar ratio of the reaction raw materials is 0.005:0.005:0.14. The product obtained by the reaction orthorhombic-LiMnO 2 Performance, effect are substantially the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com