Highly expressed recombinant gutted adenovirus containing human constant region whole antibody gene and its use
A human constant region and adenovirus technology, applied in the field of life sciences, can solve the problems that recombinant empty-shell adenoviruses have not been seen, and the therapeutic concentration of antibodies cannot be reached.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0319] Example 1. Humans carrying the humanized antibody SG-HER gene of anti-human epidermal growth factor receptor 2 (Her2), the human antibody SG-EGFR gene of anti-human epidermal growth factor receptor (EGFR), and the human anti-human CD20 Construction of expression vectors of chimeric antibody SG-CD20 gene and human-mouse chimeric antibody SG-anti HBV preS2 gene against human hepatitis B virus preS2
[0320] pShuttle-CMV was purchased from Qbiogene in the United States. It contains the human cytomegalovirus promoter (CMV IE) and SV40 poly A tailing signal. PCR technology was used to clone the human cytomegalovirus promoter (CMV IE) and SV40 poly A tailing signal. Bgl II restriction sites were added to the upstream and downstream positions, and multiple cloning sites were inserted between the human cytomegalovirus promoter (CMV IE) and the SV40 poly A tailing signal, respectively EcoRI, Sal I, Hind III, Xho I and BamH I restriction sites, the method uses positional mutation...
example 2
[0527] Example 2. Humans carrying the humanized antibody SG-HER gene of anti-human epidermal growth factor receptor 2 (Her2), the human antibody SG-EGFR gene of anti-human epidermal growth factor receptor (EGFR), and the human anti-human CD20 Construction and recombination of chimeric antibody SG-CD20 gene and anti-human-mouse chimeric antibody SG-anti HBV preS2 gene empty-shell adenovirus vector
[0528] The empty shell adenovirus helper virus (FL Helper) and 293 (203-FLPe6) containing yeast recombinase FLPe were donated by Professor Lowenstein PR of Cedars-Sinai Medical Center (Las Angeles CA 90048-1860) in the United States (see literature Umana P, Gerdes CA, Stone D, Davis JRE, Ward D, CastroMG, Lowenstein PR. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nature Biotechnology 2001 19 582-585).
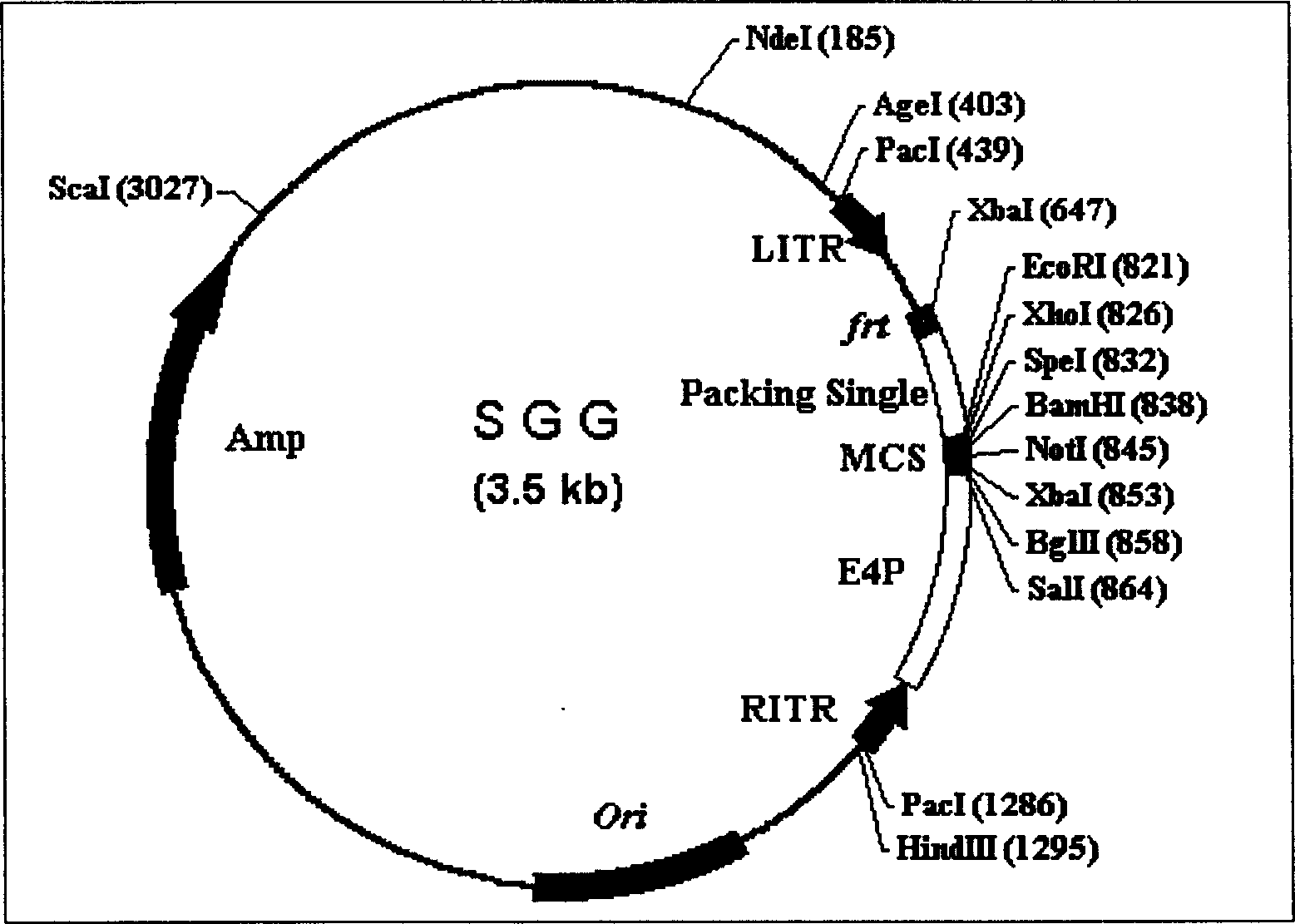
[0529] For the construction of the empty shell adenovirus vector pSGG, the following seq...
example 3
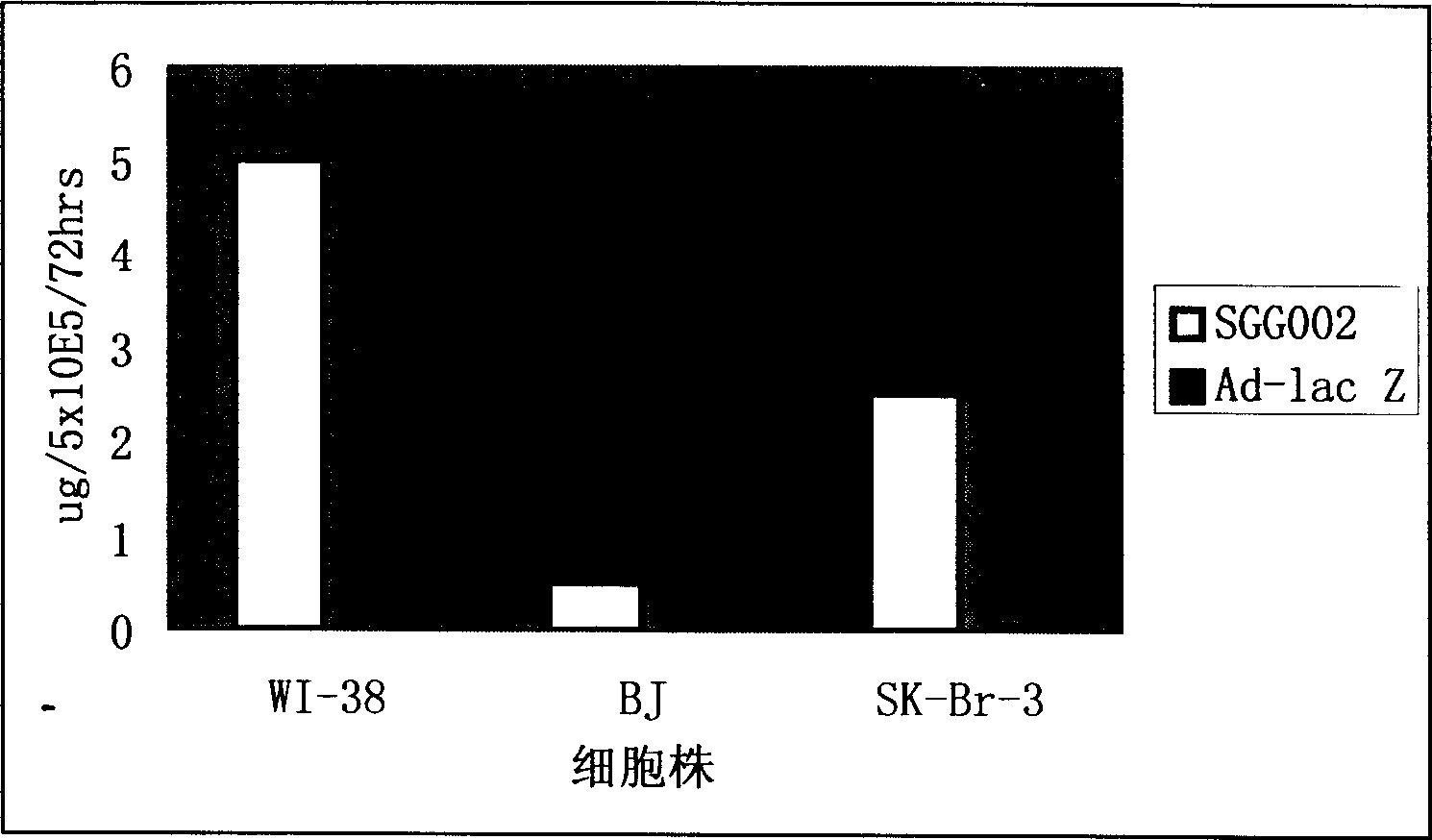
[0560] Example 3: In vitro and in vivo expression of humanized antibody by recombinant empty adenovirus (SGG002) carrying humanized antibody SG-HER gene against human epidermal growth factor receptor 2 (Her2)
[0561] The normal cell line lung fibroblast WI-38, the normal cell line lung fibroblast BJ and the human epidermal growth factor receptor 2 (Her2) positive tumor cell SK-Br-3 were purchased from ATCC Company in the United States. 5×10 5 Spread the cells / well in a 6-well plate, incubate in a 37°C incubator with 5% CO2, change 1ml of serum-free liquid the next day, then add the control adenovirus Ad5-Lac Z or anti-human epidermal growth factor receptor 2 (Her2) humanized antibody SG-HER gene recombinant empty adenovirus SGG002. Its virus load is 5×10 8 VP / well, after culturing for 90 minutes, wash twice with phosphate buffered saline (PBS) to wash away the virus, culture with 3ml of culture medium with 5% fetal bovine serum, collect the supernatant at 72 hours respectiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com