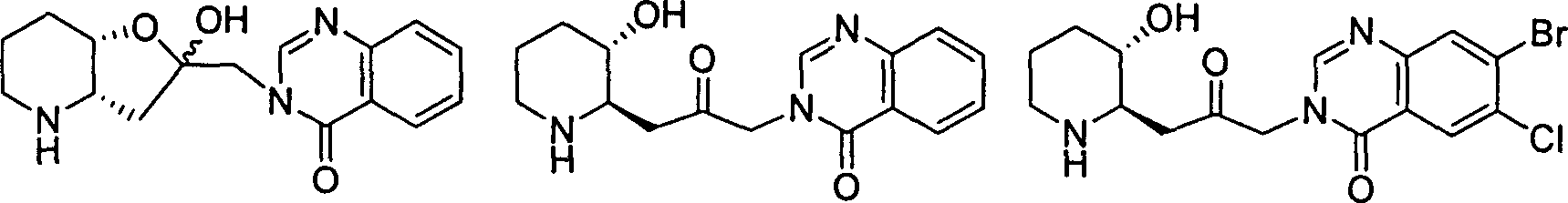
Method for synthetizing orixine and RU-19110 intermediate
A technology of RU-19110 and intermediates, applied in the direction of organic chemistry, etc., can solve the problems of low total yield and unsatisfactory selectivity of key steps, and achieve the effect of high yield and simple operation and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
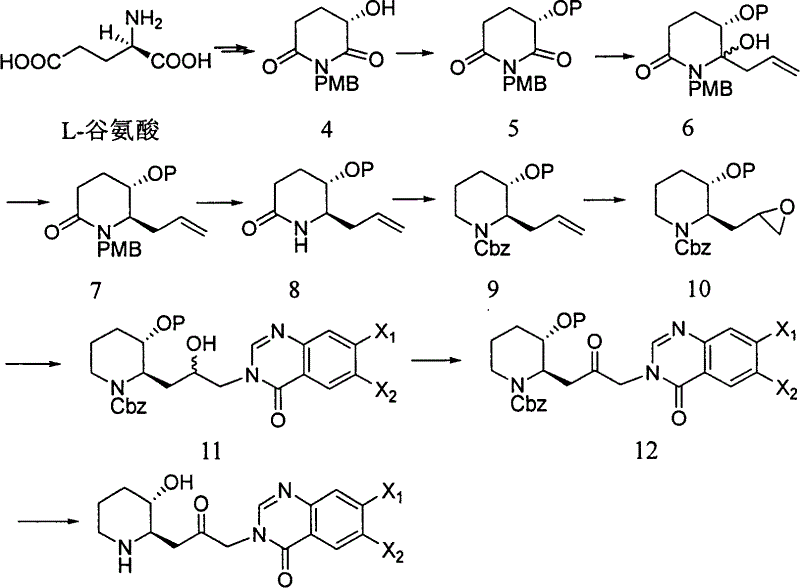
[0036] Step 1 Synthesis of (S)-3-tert-butyldimethylsilyloxy-1-(4-methoxybenzyl)-2,6-piperidinedione 5
[0037] Under the protection of nitrogen, to the solution of compound 4 (17.61 mmol) and imidazole (35.21 mmol) in dichloromethane was added a solution of tert-butyldimethylsilyl chloride (17.61 mmol) in dichloromethane (20 mL). Stir at room temperature for 6 h, add water, separate the layers, extract the aqueous phase with dichloromethane three times, wash the organic layer with saturated brine (10 mL×3) three times, dry over anhydrous sodium sulfate, and concentrate. The crude product was purified by silica gel column to obtain 5 (83%) as a colorless solid.
[0038] Step 2 Synthesis of (S)-1-(4-methoxybenzyl)-5-(tert-butyldimethylsilyloxy)-6-allyl-6-hydroxyl-2-piperidone 6
[0039]Under the protection of nitrogen, to dissolve compound 5 (2.8 mmol) in dichloromethane, slowly add a solution of AllyMgBr (8.4 mmol) in diethyl ether at -78°C, and stir for 3 h. Add 10mL NH 4 Q...
Embodiment 2
[0049] Step 1 Synthesis of (S)-3-tert-butyldimethylsilyloxy-1-(4-methoxybenzyl)-2,6-piperidinedione 5
[0050] The operation of compound 5 was the same as that in Example 1, the reaction solvent was changed to tetrahydrofuran, stirred at room temperature for 12 h, and the yield was 89%.
[0051] Step 2 Synthesis of (S)-1-(4-methoxybenzyl)-5-(tert-butyldimethylsilyloxy)-6-allyl-6-hydroxy-2-piperidone 6
[0052] Under the protection of nitrogen, a diethyl ether solution of allylmagnesium bromide was slowly added into THF dissolving compound 5 (300mg) at -78°C, and stirred for 5h. with saturated NH 4 Cl quenched. Extracted 3 times with anhydrous ether. Na 2 SO 4 After drying and concentration, the crude product was purified by silica gel (EtOAc / PE) column to obtain 6 in 76% yield and 20 / 80 regioselectivity.
[0053] Step 3 Synthesis of (S)-1-(4-methoxybenzyl)-5-(tert-butyldimethylsilyloxy)-6-allyl-2-piperidone 7
[0054] Under nitrogen protection, compound 6 was dissolved ...
Embodiment 3
[0062] Step 1 Synthesis of (S)-3-tert-butyldimethylsilyloxy-1-(4-methoxybenzyl)-2,6-piperidinedione 5
[0063] The operation of compound 5 was the same as that in Example 1, the solvent of the reaction was changed to N'N-dimethylformamide, the base used was triethylamine, stirred at room temperature for 15 h, and the yield was 55%.
[0064] Step 2 Synthesis of (S)-1-(4-methoxybenzyl)-5-(tert-butyldimethylsilyloxy)-6-allyl-6-hydroxy-2-piperidone 6
[0065] Compound 6 was prepared according to step 2 of Example 1.
[0066] Step 3 Synthesis of (S)-1-(4-methoxybenzyl)-5-(tert-butyldimethylsilyloxy)-6-allyl-2-piperidone 7
[0067] Under nitrogen protection, compound 6 was dissolved in dichloromethane, cooled to -78°C, and triethylsilane and tin tetrachloride were added. Natural stirring rose to room temperature for 16h. with saturated NaHCO 3 Quench the reaction, separate the layers, and use CH for the aqueous phase 2 Cl 2 Extracted 3 times, the organic phase was washed 3 tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com