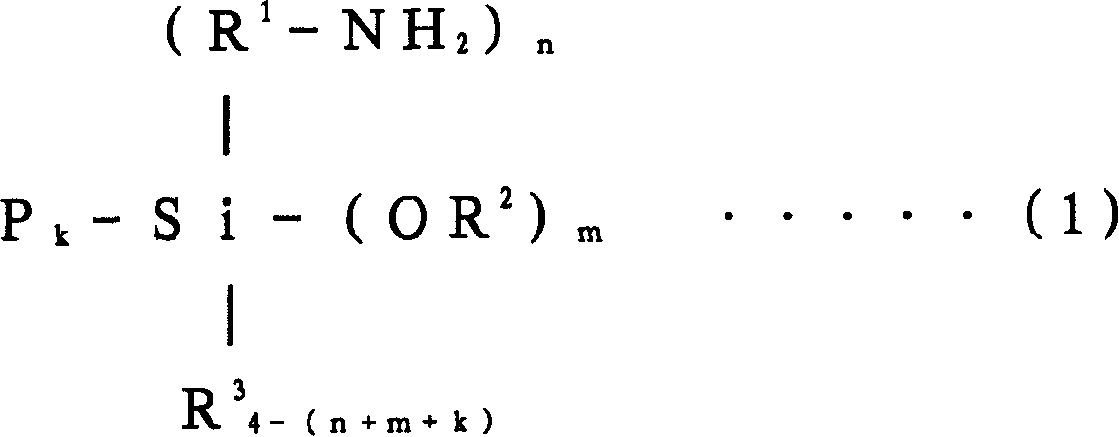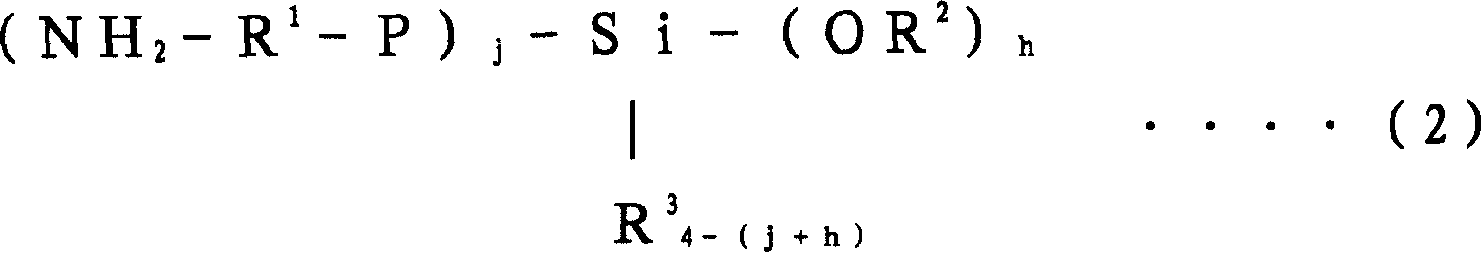Conjugated diolefin (co)polymer rubber, process for producing (co)polymer rubber, rubber composition, composite, and tire
A conjugated diene and a manufacturing method of the technology are used in the fields of rubber-inorganic compound composites, conjugated diene-based polymeric rubbers, rubber compositions, tires, conjugated diene polymeric rubbers and their manufacturing fields, and can solve the problem of drug dispersion. The performance cannot be said to be sufficient, the affinity of rubber components is not found, and a large number of drugs are dispersed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0356] Into an autoclave reactor with an internal volume of 5 liters replaced with nitrogen, 2,750 g of cyclohexane, 41.3 g of tetrahydrofuran, 125 g of styrene, and 365 g of 1,3-butadiene were charged. After adjusting the temperature of the reactor contents to 20° C., 325 mg of n-butyllithium (n-BuLi) was added to start polymerization. The polymerization was carried out under adiabatic conditions, reaching a maximum temperature of 85°C.
[0357] When the polymerization conversion rate reached 99%, 10 g of butadiene was added, and after making it polymerized for 5 minutes, 1560 mg of N,N-bis(trimethylsilyl)aminopropylmethyldimethoxysilane was added to carry out 15 minutes to react. After adding 2,6-di-tert-butyl-p-cresol to the polymer solution after the reaction, extender oil (manufactured by Fuji Industries Co., Ltd., trade name "Futsukoll Aromax (AROMAX)# 3", V.G.C=0.963) (hereinafter also referred to as "A-Oil") 187.5 g (37.5 parts relative to 100 parts of the rubber com...
Embodiment 2
[0359] In Example 1, the type and amount of additives were changed to 1.120 mg of 1-trimethylsilyl-2,2-dimethoxy-1-aza-2-silacyclopentane, and , was carried out in the same manner as in Example 1 to obtain a copolymer rubber P-2. Table 3 shows the composition and physical properties of the obtained copolymer rubber P-2.
Embodiment 3
[0361] In Example 1, except that the initiator was changed to piperidine and n-butyllithium which are secondary amines in order to introduce a tertiary amino group into the polymerization initiation terminal, the copolymerization rubber P-3 was obtained in the same manner as in Example 1. . Table 3 shows the composition and physical properties of the obtained copolymer rubber P-3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| oil absorption | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com