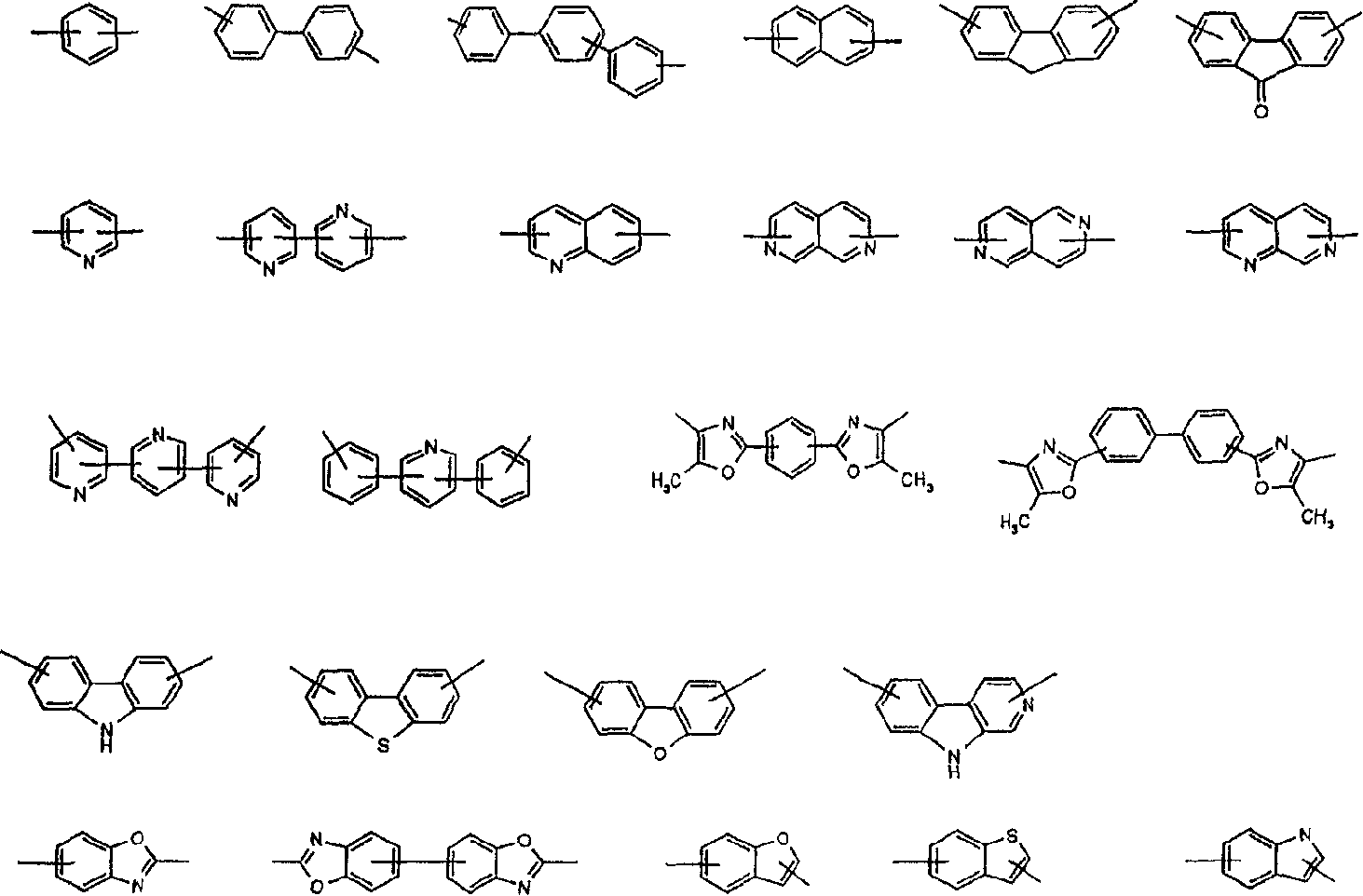
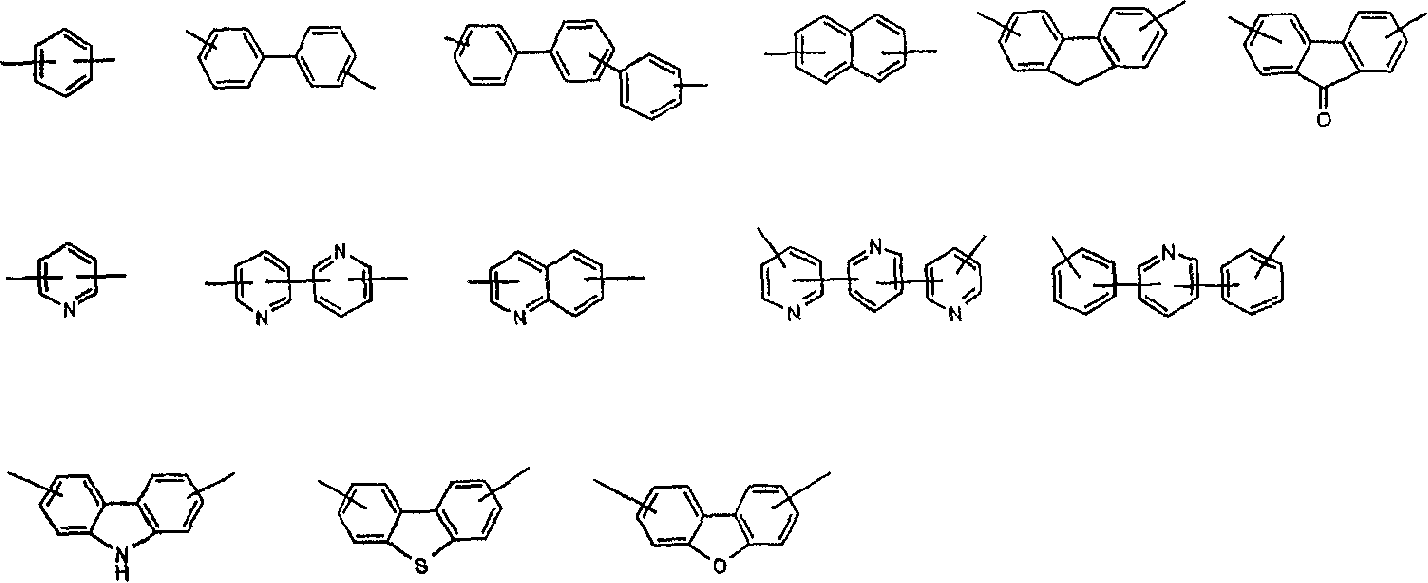
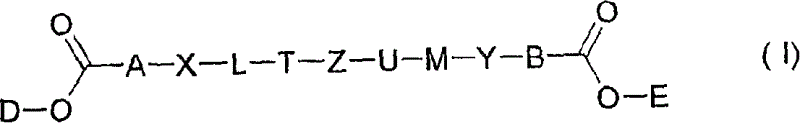Novel compounds, their preparation and use
A compound and mixture technology, applied in the fields of dicarboxylic acid derivatives, their preparation and therapeutic applications, can solve the problems of inability to overcome macrovascular complications and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0532] Embodiment 1 (general method A)
[0533] (E)(E)(S)(S)2-Ethoxy-3-{4-[5-(4-{5-[4-(2-ethoxy-2-ethoxycarbonylethyl) )-phenoxy]-pent-3-en-1-ynyl}-phenyl)-pent-2-en-4-ynyloxy]-phenyl}-propionic acid ethyl ester
[0534]
[0535] Steps A-B:
[0536] To a solution of 1,4-diiodobenzene (1.32 g, 4.0 mmol) in diisopropylamine (12 mL) was added copper(I) iodide (60 mg, 0.3 mmol) and tetra (Triphenylphosphine)palladium (80 mg, 0.07 mmol). After stirring the mixture for 1 h, a solution of 2-penten-4-yn-1-ol (1.0 g, 12.0 mmol) in diisopropylamine (7 mL) was added. After stirring at 60 °C for 8 h under nitrogen atmosphere, the reaction mixture was filtered and the filtrate was evaporated to dryness. The product was purified by flash chromatography using toluene / ethyl acetate (2:1) gradually transitioning to ethyl acetate as eluent to give 520 mg (55%) of (E)(E)5-[4-(5-hydroxy -pent-3-en-1-ynyl)-phenyl]-pent-2-en-4-yn-1-ol.
[0537] 1 H NMR (CDCl 3 ): δ1.47(2H, bs), 4.28(2H, ...
Embodiment 2
[0541] Embodiment 2 (general method E)
[0542] (E)(E)(S)(S)3-{4-[5-(4-{5-[4-(2-carboxy-2-ethoxy-ethyl)-phenoxy]-pentane -3-en-1-ynyl}-phenyl)-pent-2-en-4-ynyloxy]-phenyl}-2-ethoxy-propionic acid
[0543]
[0544] Step A:
[0545] To (E)(E)(S)(S)2-ethoxy-3-{4-[5-(4-{5-[4-(2-ethoxy-2-ethoxycarbonylethyl Base)-phenoxy]-pent-3-en-1-ynyl}-phenyl)-pent-2-en-4-ynyloxy]-phenyl}-propionic acid ethyl ester (Example 1 ) (88 mg 0.13 mmol) in THF (3 mL) and ethanol (3 mL) was added 1N sodium hydroxide (2 mL). After stirring at room temperature for 1 h, the reaction mixture was concentrated under reduced pressure, and water and 1N hydrochloric acid were added until the pH value was 1. The product was extracted three times with dichloromethane, and the combined organic phases were washed with magnesium sulfate (MgSO 4 ), filtered and concentrated under reduced pressure to give the title compound as a crystalline product. The product was recrystallized from ethyl acetate and petrole...
Embodiment 3
[0547] Embodiment 3 (general method A)
[0548] (E)(E)3-Chloro-4-(5-4-[5-(3-chloro-4-ethoxycarbonylmethyl-phenoxy)-pent-3-en-1-ynyl ]-phenyl}-pent-2-en-4-ynyloxy)-phenyl]-ethyl acetate
[0549]
[0550] Step C:
[0551] Under nitrogen atmosphere, azodicarboxylic acid dipiperidine amide (504mg, 2.0mmol) was added to tributylphosphine (404mg, 2.0mmol), (3-chloro-4-hydroxybenzene base) ethyl propionate (322 mg, 1.5 mmol) and (E)(E) 5-[4-(5-hydroxy-pent-3-en-1-ynyl)-phenyl]-pent-2-ene -4-Alkyn-1-ol (Example 1, Steps A-B) (120 mg, 0.5 mmol) in a stirred solution in dry THF (25 mL). After 1 h, the reaction mixture was filtered, and the filtrate was concentrated under reduced pressure. The crude product was purified by flash chromatography using a gradual transition from toluene to toluene / ethyl acetate (1:1) as eluent. The product was recrystallized from ethyl acetate to give 150 mg (48%) of the title compound.
[0552] 1 H NMR (CDCl 3 ): δ1.24(6H, t), 3.53(4H, s), 4.15(4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com