Use and constructing plan of anticancer recombined gland virus with tumour CHK1 as target of medicine
A technology of recombinant adenovirus and construct, which is applied in the field of medical genetic engineering, can solve the problems of limited therapeutic effect, lack of tumor specificity, and no therapeutic target gene, and achieve the effect of avoiding attack and increasing copy number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Construction of pXC1 series mutants (Δ923-946pXC1)
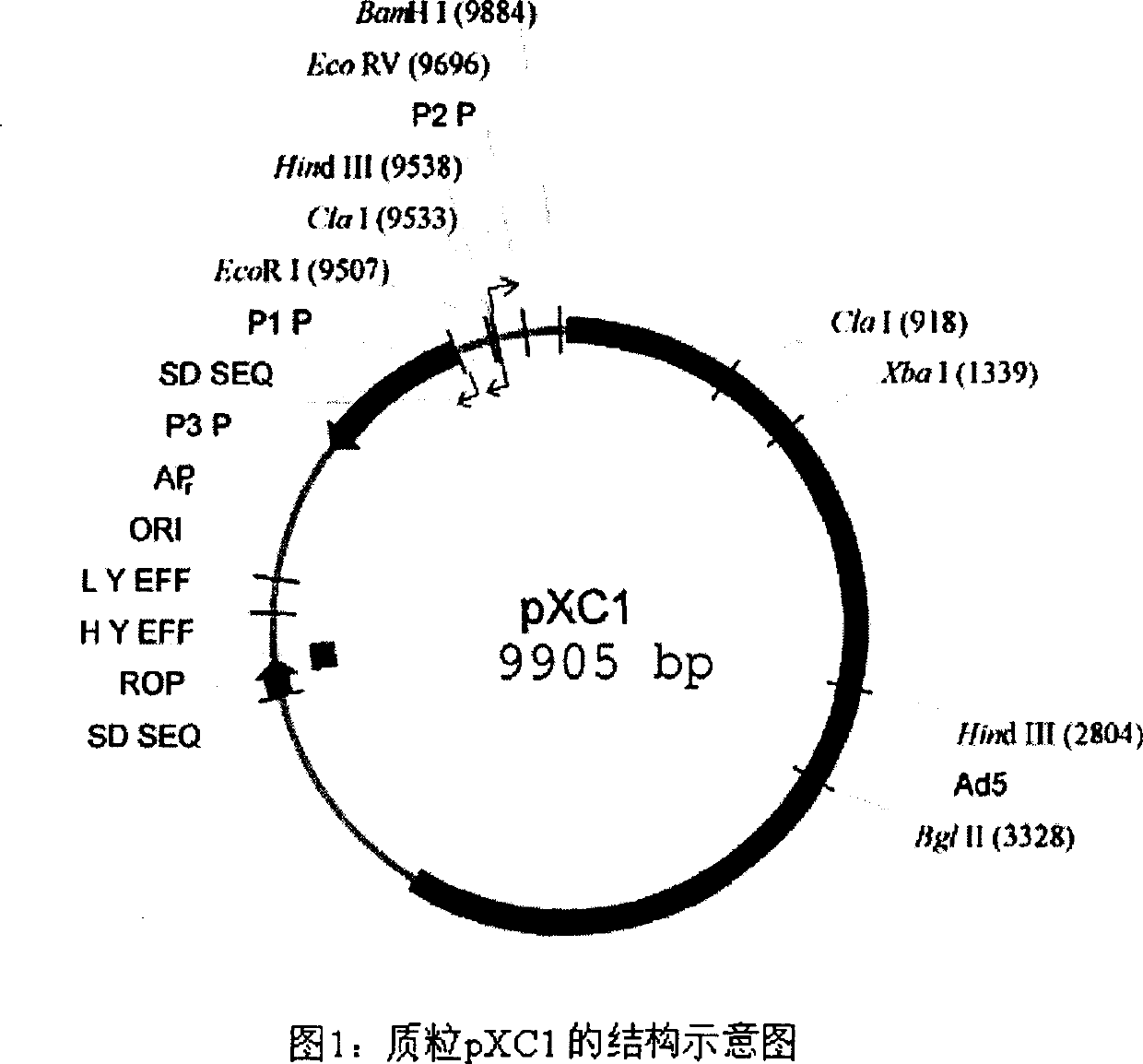
[0031] 1. pXC1 was purchased from Microbix Biosystem Inc. (Toronato, Ontario, Canada, catalog number: PD-01-03). The plasmid contains the 21-5790nt sequence of human adenovirus type 5 (ADV5). The plasmid structure is shown in Figure 1 194-358nt of the plasmid is the ADV5 adenovirus packaging signal, the sequence 560-1112nt, 1229-1545nt is the coding sequence of functional protein E1A, 9883-9888nt contains the BamHI restriction site, and 1338-1343nt contains the XbaI restriction site.
[0032] 2. Deletion of 923-946nt by 3 times PCR.
[0033] (1) Acquisition of Fragment 1: Primer 1 = 5'-CG GGA TCC GGG CCC CCA TTT CC-3' (equivalent to 9883-9902nt, the underlined part is the BamHI restriction site); primer 2 = 5'- GTC ACT GGG TGG ATC GAT CAC CTC CGG TAC-3' (equivalent to 922-905nt, the underlined part is complementary to primer 3);
[0034] Use pXC1 as a template for PCR reaction, the total volume of t...
Embodiment 2
[0042] Example 2: Construction of Δ923-946ADV5 recombinant adenovirus
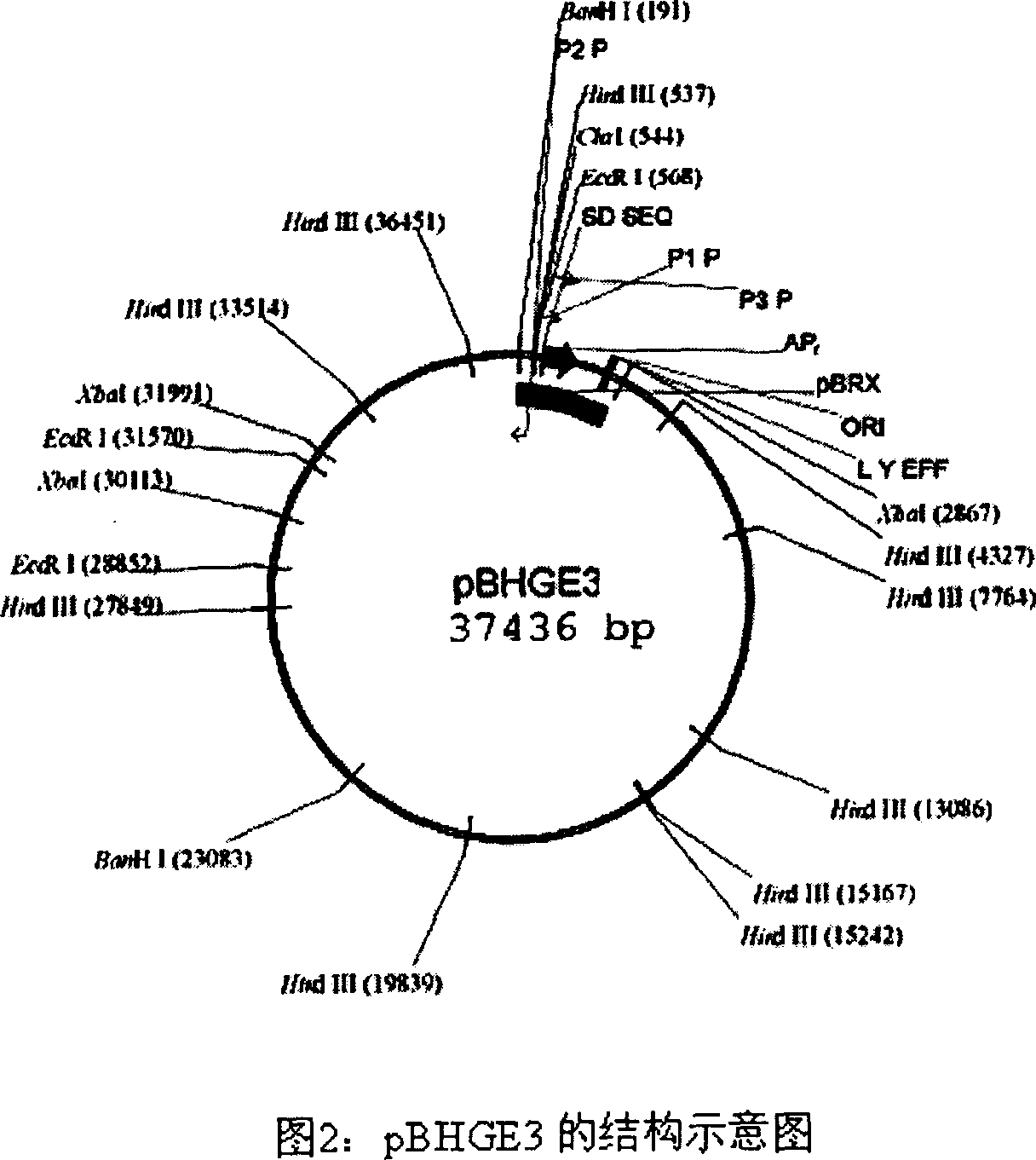
[0043] 1. pBHGE3 was purchased from Microbix Biosystem Inc. (Toronato, Ontario, Canada, catalog number: PD-01-12). This plasmid contains all genome sequences except the ADV5 packaging signal (194-358nt), and between 194-358nt An artificial sequence is inserted between them, the full length is 37436bp, and its structure is shown in Figure 2.
[0044] When pBHGE3 is obtained from Microbix Biosystem Inc., the total amount is 10 μg. First, electroporate into competent bacteria and pick positive clones. Since the plasmid fragment is very long, CsCl is needed 2 -Eb The plasmid was purified by ultracentrifugation according to the method in the book "Molecular Cloning".
[0045] 2. Homologous recombination method to obtain Δ923-946ADV5 recombinant adenovirus construct, the method is as follows:
[0046] (1), plant 7.5×10 in a 15cm petri dish 5 293 cells (ATCC, U.S.A, catalog number: CRL-1573), the culture mediu...
Embodiment 3
[0062] Example 3: Construction of the subcloning vector pCDNA3.1-ΔE3 in the ADV5 E3 region
[0063] 1. The full name of the ADV5 E3 region is the early region 3 of ADV5. Driven by the endogenous promoter, this region encodes 7 proteins. The sequence, structure and function are as follows (see Figure 3): 12.5k, 27858-28179nt, function Unidentified; 6.7k, 27547-28736nt, function unknown; gp19k, 28735-29215nt, binds MHC class I antigens, inhibits their presentation to the cell surface, resulting in evasion of CTL clearance; ADP, 29419-29770nt, lyses cells, releases adenovirus ; RIDα, 29784-30057nt, forms a complex with RIDβ, prevents the lysis of TNF, and clears the FAS antigen; RIDβ, 30062-30458nt; 14.7k, 30453-30837, inhibits the lysis of TNF.
[0064] The purpose of this experiment is to delete the 28530-29355nt region of the E3 region and insert an exogenous therapeutic gene into the E36.7k / gp 19k region of the recombinant adenovirus.
[0065] 2. Delete 28532-29360nt of ADV5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com