Conditionally active chimeric antigen receptors for modified t-cells
a technology of chimeric antigen receptors and t-cells, which is applied in the field of protein evolution to achieve the effects of increasing activity, reducing activity, and increasing binding activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of scFv Conditionally Active Antibodies
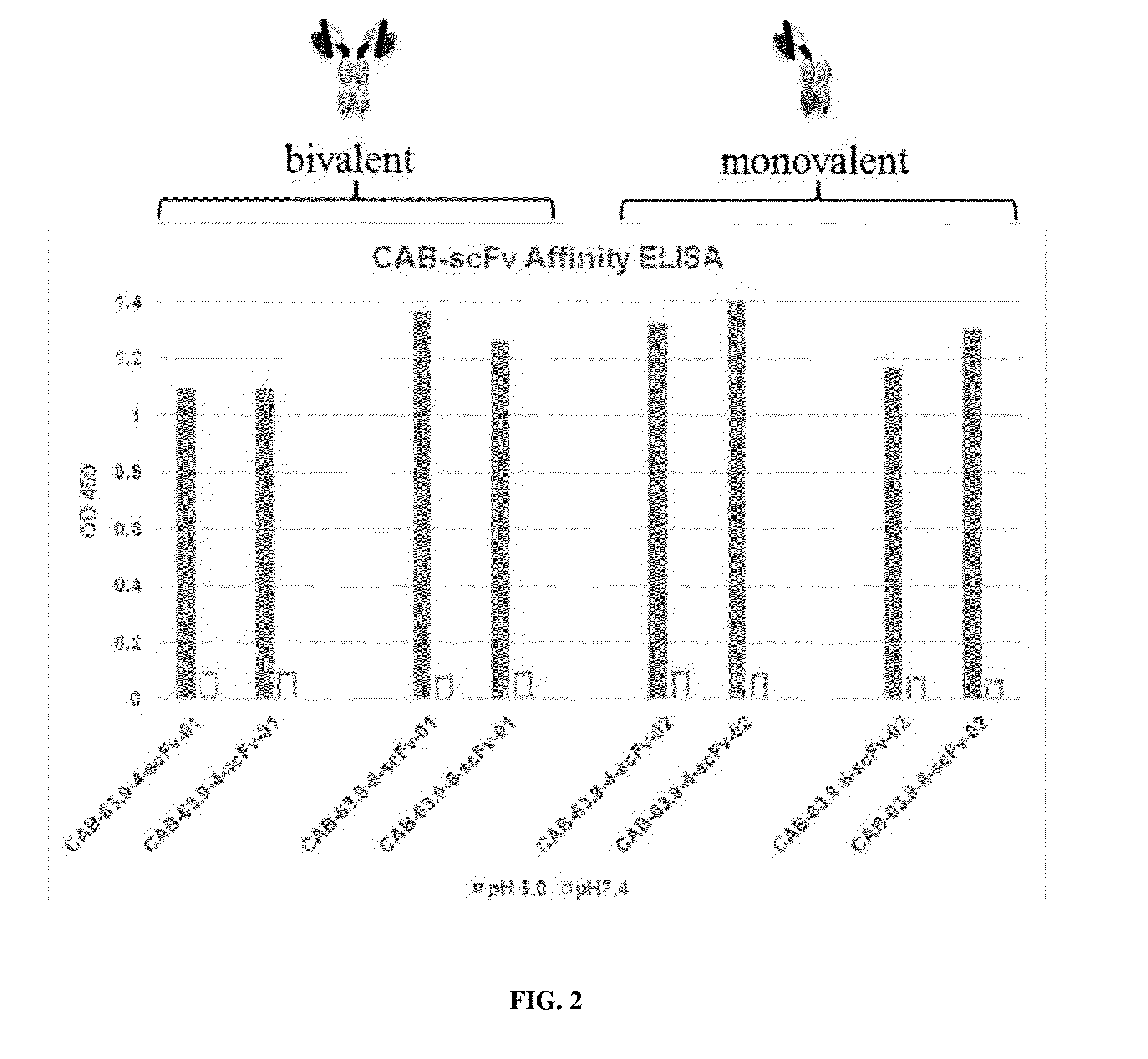
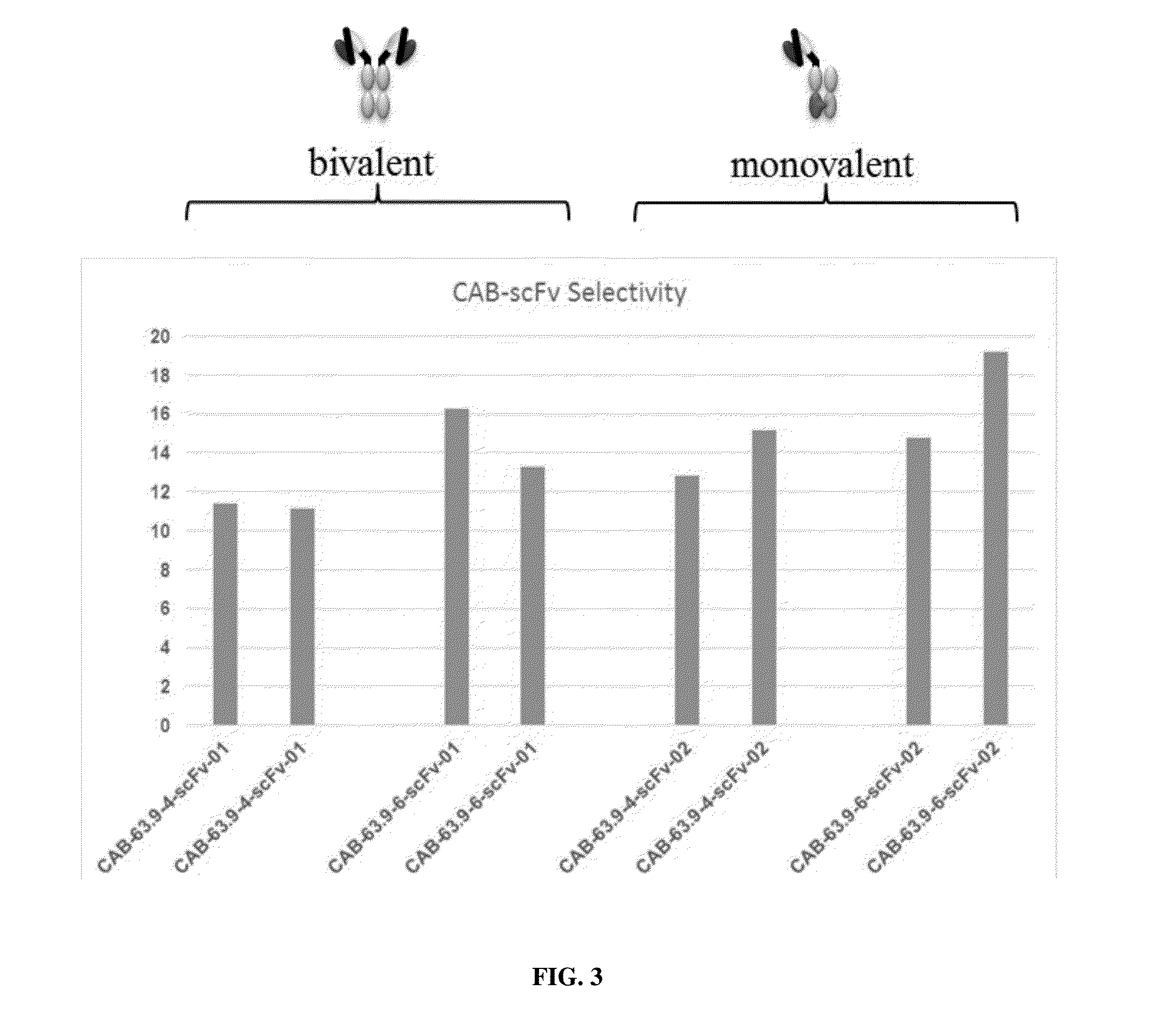
[0365]Two conditionally active antibodies (CAB-scFv-63.9-4 and CAB-scFv-63.9-6) for a drug target antigen X1 were expressed as homodimers with wild type human IgG1 Fc (resulting in bivalent antibodies CAB-scFv-63.9-4-01 and CAB-scFv-63.9-6-01 in FIGS. 2-3), as well as heterodimers in the knob-in-hole system resulting in a monovalent scFv (resulting in monovalent antibodies scFv CAB-scFv-63.9-4-02 and CAB-scFv-63.9-6-02 in FIGS. 2-3).
[0366]The binding affinities of these antibodies to the drug target antigen X1 at pH 6.0 and pH 7.4 were measured by the ELISA assay. As show in FIG. 2, the scFv antibodies showed affinities to drug target antigen X1 at both pH 6.0 and pH 7.4, which were comparable to the full bivalent antibodies. Further, the selectivity of these scFv antibodies at pH 6.0 over pH 7.4 as shown in FIG. 3 was also comparable to the full bivalent antibodies. This example demonstrated that the conditionally active antibodies ...
example 2
scFv Antibodies Against Target Antigen X1 for Constructing CAR-T Cells
[0367]Conditionally active antibodies for the drug target antigen X1 were generated by simultaneously screening for selectivity and affinity, as well as expression level at both pH 6.0 and pH 7.4, in accordance with one embodiment of the present invention. The screening was done in serum using a FLAG tag because there were human antibodies in the serum which might cause false positives for the screening. The screening buffer was a carbonate buffer (krebs buffer with ringer—standard buffer but different from PBS). The generated conditionally active antibodies were found to have a higher affinity to the drug target antigen X1 at pH 6.0 but lower affinity to the same drug target antigen X1 at pH 7.4, both in comparison with the wild-type antibody. Further, these conditionally active antibodies all have high expression levels as shown in Table 2 below, with column “Clone” showing the antibodies and the expression leve...
example 3
CAR-T Cells with a Conditionally Active scFv Antibody Against Target Antigen X1
[0377]A conditionally active scFv antibody against target antigen X1 was used to construct a CAR molecule. T cells were transduced with the CAR molecule such that the T cells expressed the CAR molecule (CAR-T cells). CHO cells expressing target antigen X1 (CHO-63 cells) or regular CHO cells that do not express target antigen X1 (CHO cells) were separately treated with the CAR-T cells. Non-transduced T-cells (without the CAR molecule) were used as a control (FIGS. 8A-8B).
[0378]Referring to FIG. 8A, CHO cells that do not express the target antigen X1 were treated with the CAR-T cells and non-transduced T-cells. There was no significant difference between the two treatments, indicating no cytotoxicity of the CAR-T cells to the CHO cells. Referring to FIG. 8B where CHO cells expressing target antigen X1 (CHO-63) were similarly treated, the CAR-T cells with a conditionally active antibody against target antige...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com