Method for producing l-lysine
An amino acid and nucleotide technology, applied in biochemical equipment and methods, botanical equipment and methods, applications, etc., can solve problems such as improving growth, no L-lysine biosynthesis genes are reported, and achieve yield improvement. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
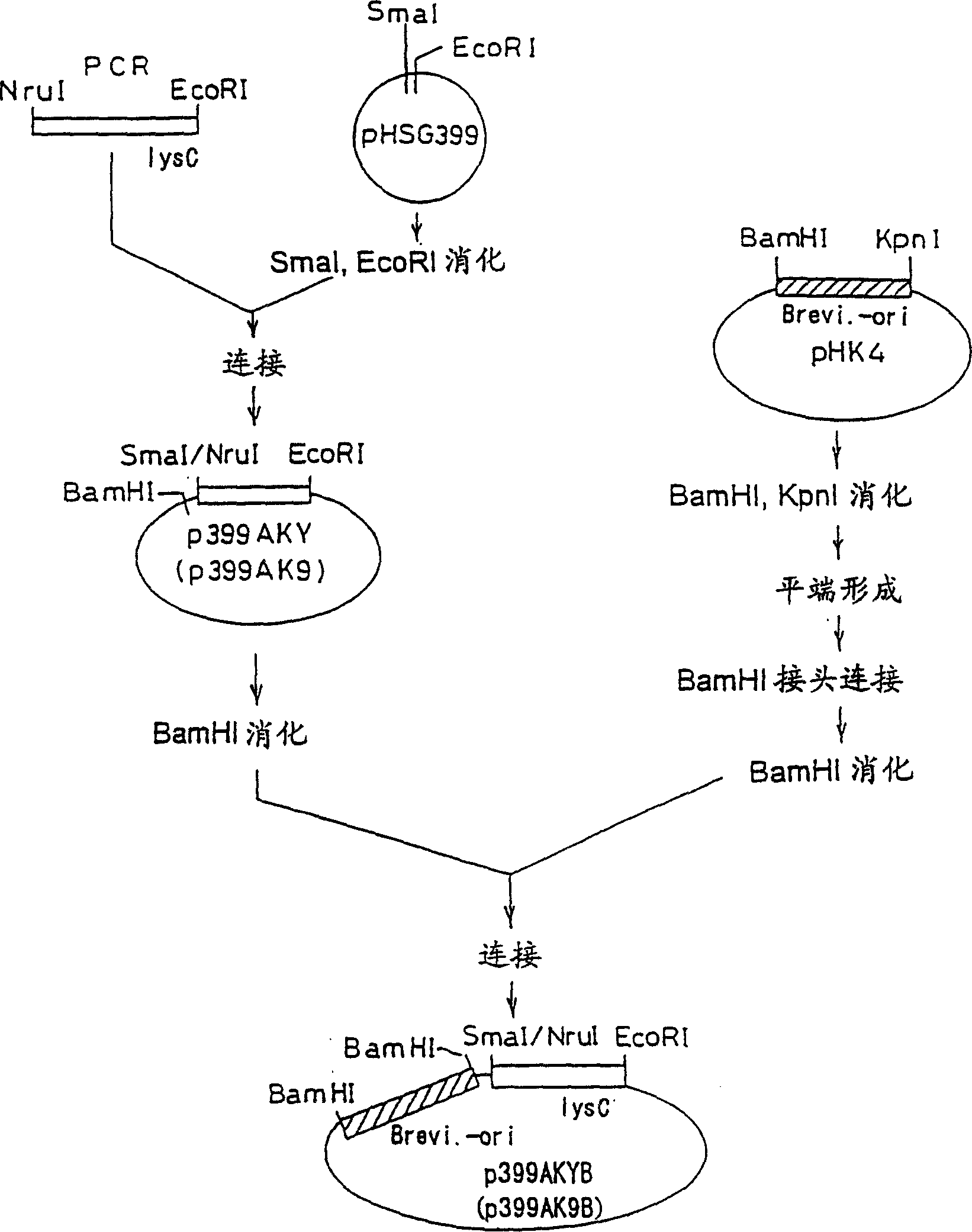
[0122] Example 1: Preparation of wild-type lysC gene and mutant lysC gene from Brevibacterium lactofermentum
[0123] 1 Preparation of wild-type and mutant lysC and preparation of plasmids containing them
[0124] The Brevibacterium lactofermentum ATCC 13869 strain and the L-lysine-producing mutant strain AJ3445 (FERM P-1944) obtained from the ATCC 13869 strain by mutation treatment were used as chromosomal DNA donors. The AJ3445 strain has undergone mutations that alter lysC to substantially desensitize the synergistic inhibition by lysine and threonine (J. Biochem., 68, 701-710 (1970)).
[0125] A DNA fragment containing lysC was amplified from chromosomal DNA by the PCR method (polymerase chain reaction; see White, T.J. et al., Trends Genet., 5, 185 (1989)). A 1,643bp region encoding lysC was added based on the known sequence of Corynebacterium glutamicum (see Molecular Microbiology (1991), 5(5), 1197-1204; and Mel.Gen.Genet. (1990), 224, 317-324) to synthesize 23-mer and...
Embodiment 2
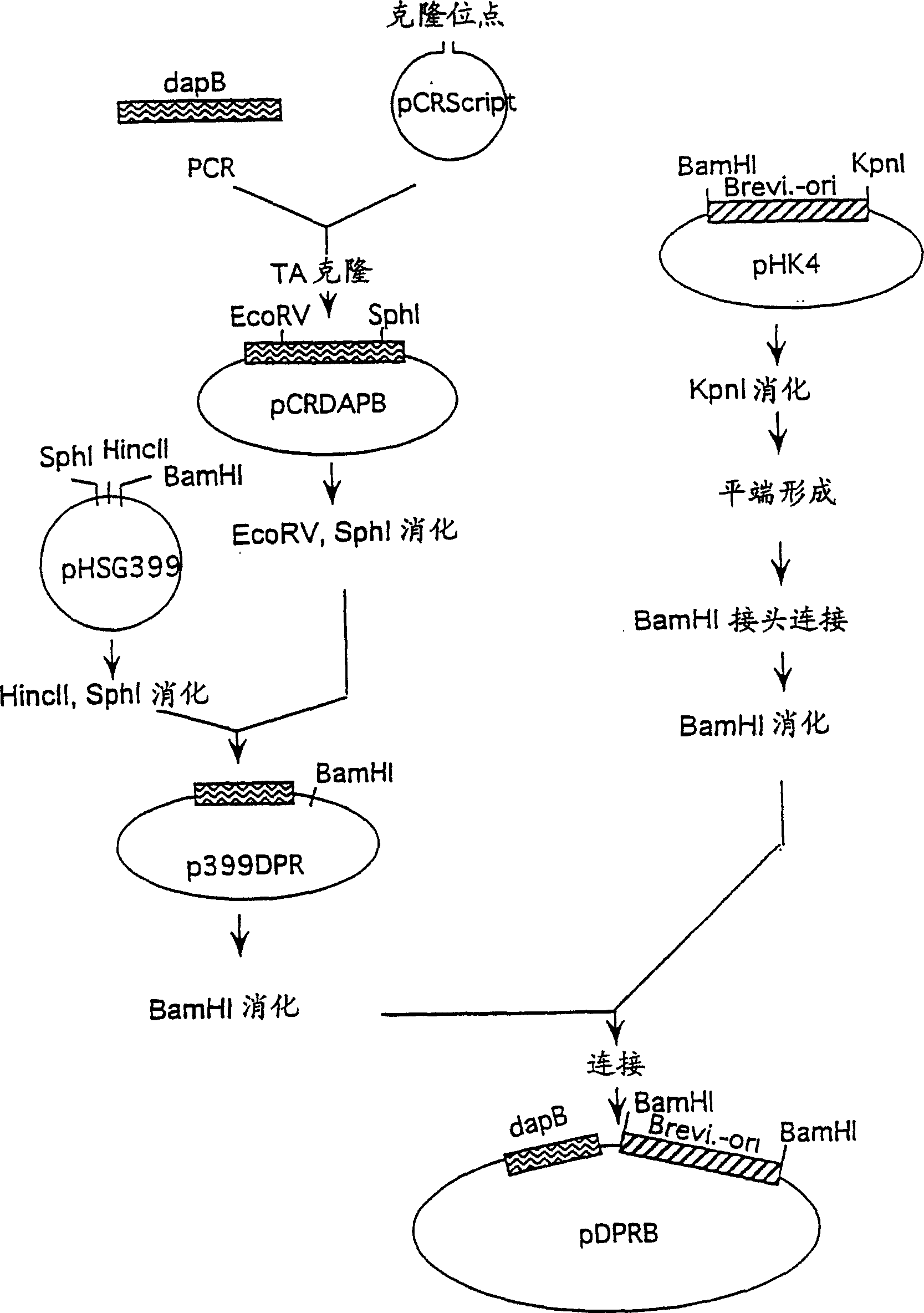
[0136] Example 2: Preparation of dapB from Brevibacterium
[0137] 1 Preparation of dapB and construction of a plasmid containing dapB
[0138] Brevibacterium lactofermentum wild-type strain ATCC 13869 was used as the chromosomal DNA donor. Chromosomal DNA was prepared from the ATCC 13869 strain according to an ordinary method. A DNA fragment containing dapB was amplified from chromosomal DNA according to PCR. As for the DNA primers used for amplification, in order to amplify the 2.0 kb region encoding DDPR, based on the known sequence of Brevibacterium lactofermentum (see Journal of Bacteriology, 175(9), 2743-2749(1993)), respectively 23-mer DNA (with the nucleotide sequences shown in SEQ ID NO: 8 and 9 in the Sequence Listing) was synthesized, and DNA synthesis and PGR were performed in the same manner as described in Example 1. pCR-Script (manufactured by Invitrogen) was used as a cloning vector for amplifying a gene fragment of 2,001 bp, which was ligated with the ampli...
Embodiment 3
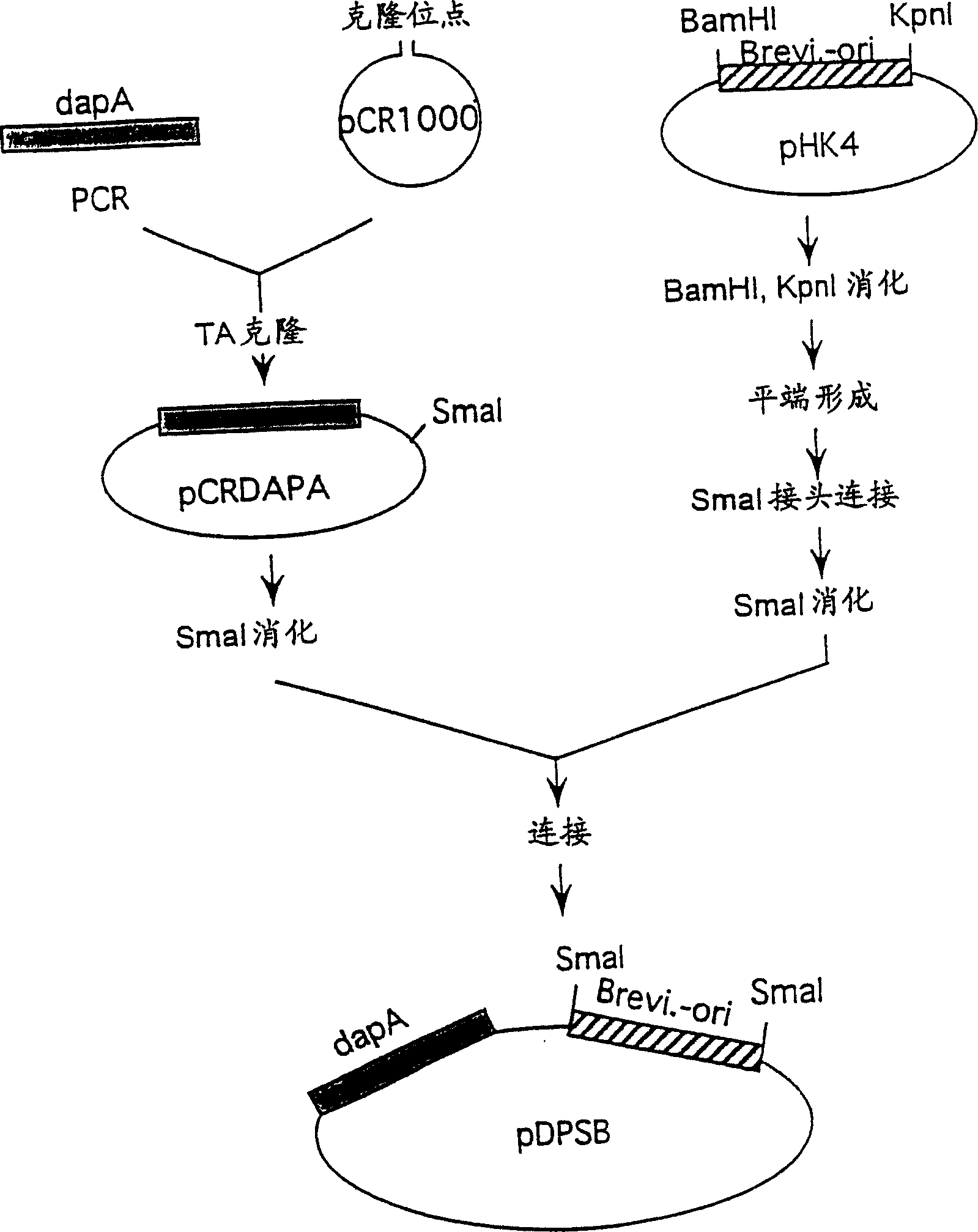
[0144] Example 3: Preparation of dapA from Brevibacterium
[0145] 1 Preparation of dapA and construction of a plasmid containing dapA
[0146] Brevibacterium lactofermentum wild-type strain ATCC 13869 was used as the chromosomal DNA donor. Chromosomal DNA was prepared from the ATCC 13869 strain according to an ordinary method. A DNA fragment containing dapA was amplified from chromosomal DNA according to PCR. As for the DNA primers used for amplification, in order to amplify the region of 1.5kb encoding DDPS, based on the known sequence of Corynebacterium glutamicum (Nucleic Acid Research, 18 (21), 6421 (1990); EMBL deposit number X53993 ) to synthesize 23 strands of DNA respectively having the nucleotide sequences shown in SEQ ID NO: 12 and 13 in the sequence listing, and DNA synthesis and PCR were carried out in the same manner as described in Example 1. pCR-1000 (manufactured by Invitrogen, see Biotechnology, 9, 657-663 (1991)) was used as a cloning vector for amplifyin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com