Hydrogel-driven drug dosage form
A pharmaceutical dosage form and dosage form technology, which is applied in the field of hydrogel-driven pharmaceutical dosage forms, can solve the problems of increasing the dosage form of drugs, and achieve rapid delivery and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] After the preparation of the core 12, the coating 18 process is carried out. The coating 18 should not only have high enough water permeability to release the drug within the desired time, but also have high strength and be relatively easy to prepare. Water permeability controls the rate at which water enters the core, thereby controlling the rate at which the drug is released into the environment of use. If high doses of low solubility drugs are required, the combination of low solubility and high doses requires a highly permeable coating to achieve drug release while maintaining an appropriate dosage form size. The need for high strength is to ensure that the coating will not be damaged when the core is wetted and swelled, resulting in uncontrolled release of the drug. Application of the coating should be relatively easy. Furthermore, the coating should be insoluble and non-erodible during release of the drug-containing composition, i.e., the coating should be suffi...
Embodiment 1
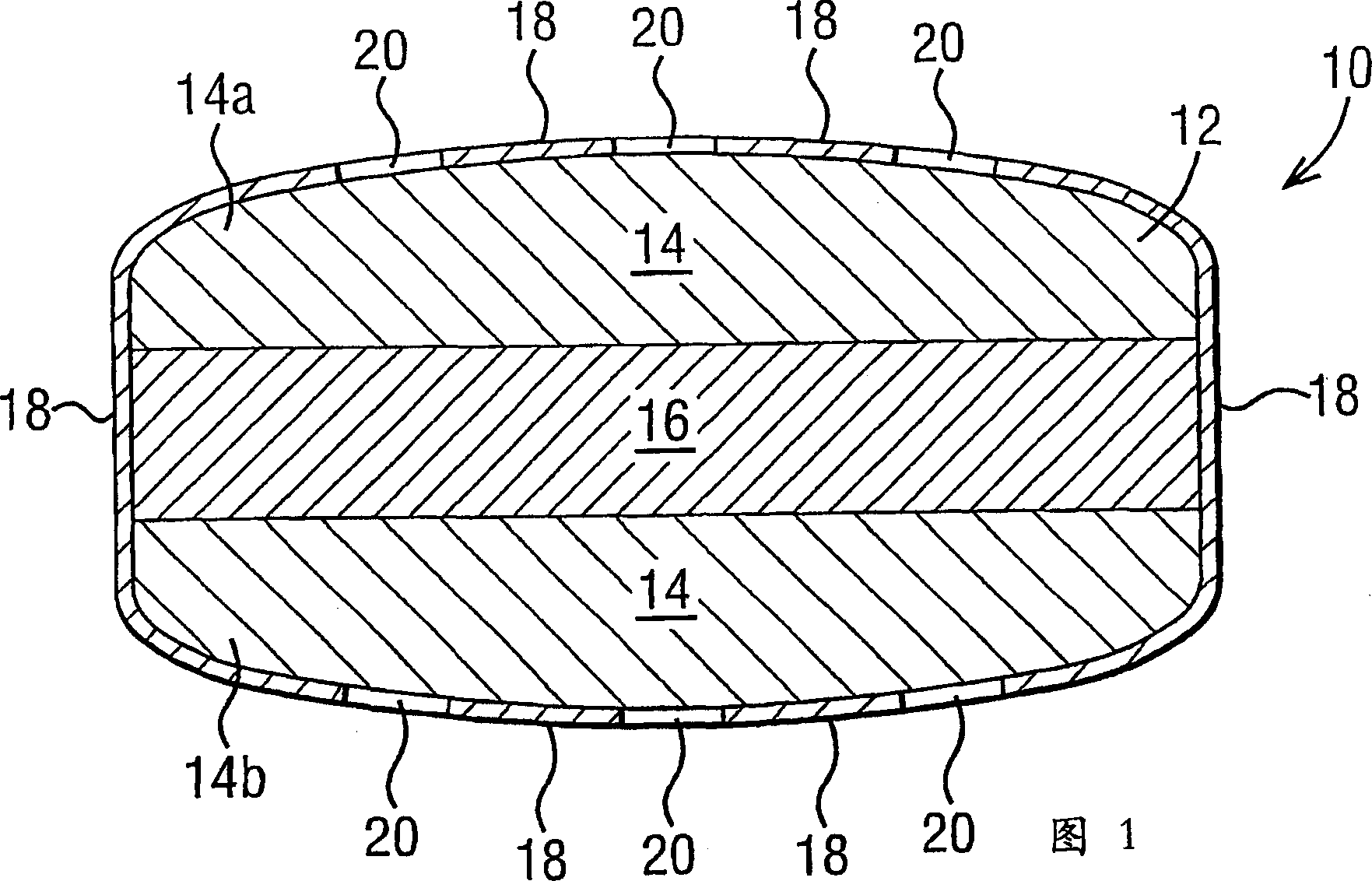
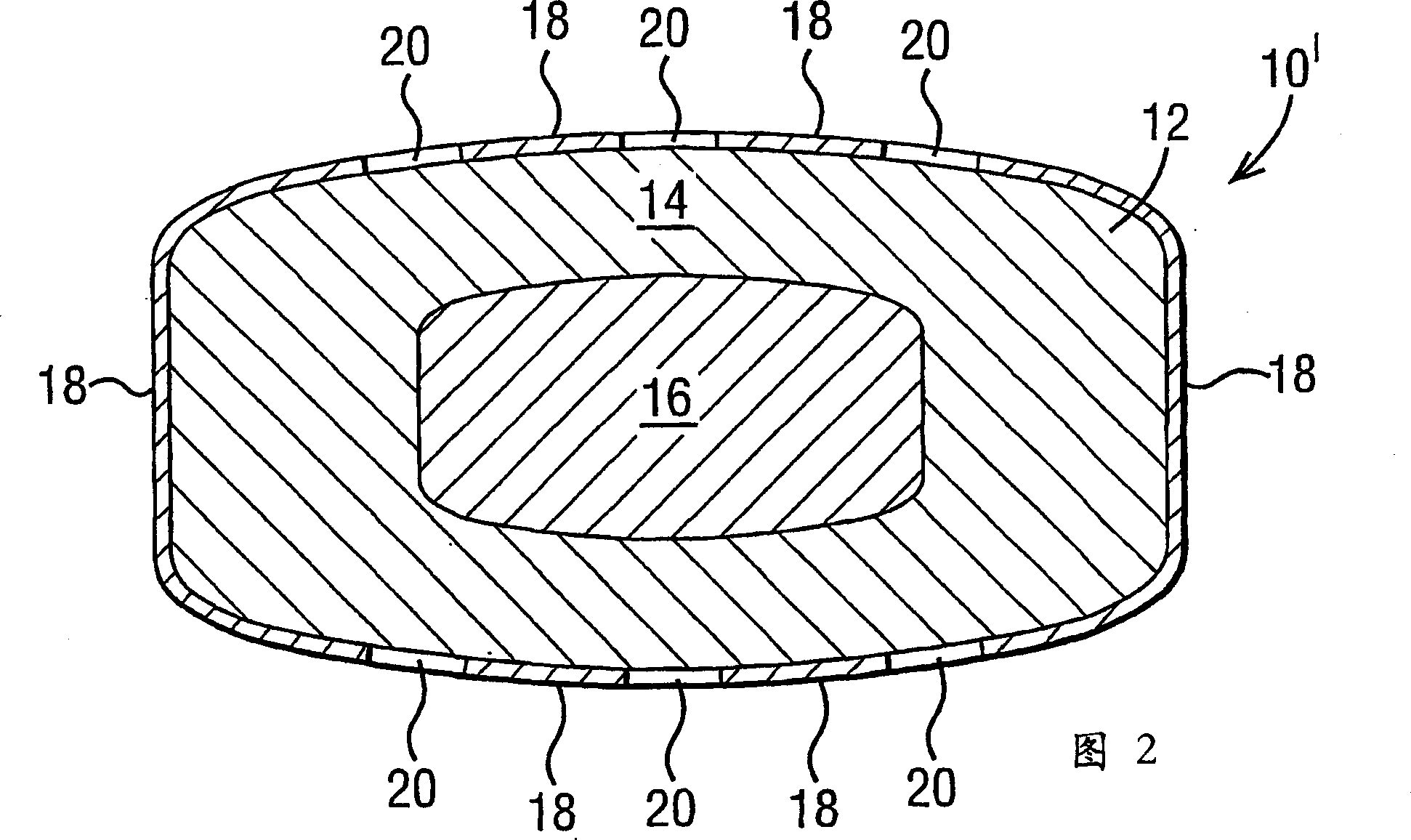
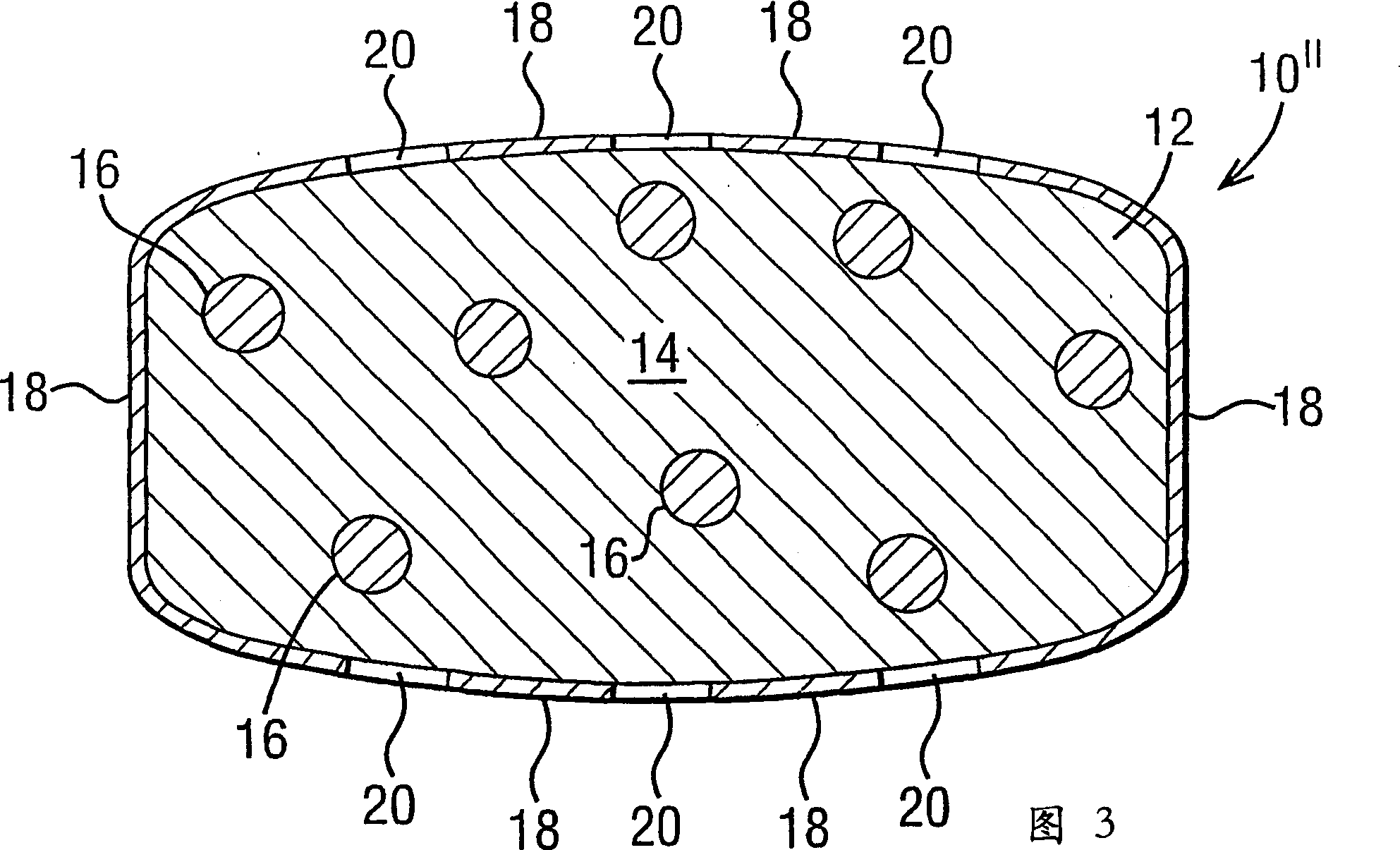
[0117] The three-layer geometry of a typical dosage form of the invention is depicted in Figure 1 of the accompanying drawings. The composition of the three-layer core is that the drug-containing composition is uniformly distributed on the top and bottom of the tablet layer and the water-swellable composition is contained in the middle layer.
[0118] To form the medicated composition, the following was wet granulated (see Table A): 35% by weight of 1-[4-ethoxy(6,7-dihydroxy-1-methyl-7-oxo-3-propane Base-1H-pyrazolino[4,3-d]pyrimidin-5-yl)benzenesulfonyl]-4-methylpiperazine citrate, also known as sildenafil (sildenafil) citrate ( Herein referred to as drug 1) with a solubility of about 20 g / ml at pH=6, 30 wt% xylitol (commercial name XYLITAB200), 29 wt% PEO with an average molecular weight of 600,000 Daltons, 5 wt% sodium starch glycolate (commercial name EXPLOTAB), and 1 wt% magnesium stearate. The ingredients of the drug-containing composition, in the absence of magnesium ...
Embodiment 2A-2D
[0126] These examples describe the release of different drugs of the invention from three-layer tablets. For the tablet of Example 2A, the drug-containing composition contains 28 wt% of sertraline hydrochloride (drug 2), the solubility of which is about 0.2 mg / ml at pH=7, 37 wt% of XYLITAB 200, and an average molecular weight of 29 wt% 600,000 Daltons of PEO, 5 wt% of EXPLOTAB, and 1 wt% of magnesium stearate. The ingredients of the medicinal composition, except the magnesium stearate, are first mixed in a TURBULA mixer for 20 minutes. Next, grind with a hammer mill and pass through a 0.065-inch screen. The materials were mixed for an additional 20 minutes in the TURBULA mixer. Additional magnesium stearate was added and mixing continued for 4 minutes.
[0127] To prepare the water-swellable composition, the following materials were mixed: 72.5% by weight of EXPLOTAB, 25% by weight of microcrystalline cellulose (AVICEL PH102), and 2.5% by weight of magnesium stearate. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com